Articles
- Page Path
- HOME > J Korean Acad Nurs > Volume 51(2); 2021 > Article
- Research Paper Impact of Obesity on Urinary Tract Infections in Korean Adults: Secondary Data Analysis Using Community-Based Cohort Study
- Seung Hee Seo, Ihn Sook Jeong, Eun Joo Lee
-
Journal of Korean Academy of Nursing 2021;51(2):150-161.
DOI: https://doi.org/10.4040/jkan.20228
Published online: April 30, 2021
2College of Nursing, Pusan National University, Yangsan
3Department of Nursing, Dong-Eui University, Busan, Korea
Abstract
Purpose
This study investigated the incidence of urinary tract infection (UTI) in community-dwelling adults and identified the association between obesity and UTI.
Methods
The participants were 4,926 adults aged over 40 years who had no UTIs at the baseline survey of the Korean Genome Epidemiology Study. Obesity was defined according to the cirtieria of Korean Society for the Study of Obesity using body mass index (BMI) data. UTI was defined as those who had self-reported UTI or had either nitrite, or both leukocytes and blood in the urine dipstick test. Hazard ratio (HR) and 95% confidence interval (CI) were calculated using a multivariate Cox proportional hazards regression analysis to identify the association between the obesity and UTI.
Results
The incidence proportion of UTI was 5.1%, and the incidence density per 1,000 person-years was 25.5. After controlling general characteristics, people with BMI ≥30.0 kg/m2 remained 1.66 times (HR = 1.66, 95% CI = 1.06~2.60; p < .05) more likely to have UTI than those with normal weight. This trend was also present in men or people aged ≥ 60 years. Among women aged ≥ 60 years, people with BMI ≥ 30.0 kg/m2 were 1.98 times (HR = 1.98, 95% CI = 1.01~3.86; p < .05) more likely to have UTI than those with normal weight.
Conclusion
The BMI ≥ 30.0 kg/m2 is a risk factor of UTIs in Korean adult men over 40 years and women aged ≥ 60 years. It is necessary to emphasize the importance of obesity management to men or women aged ≥ 60 years, specifically.
Published online Apr 30, 2021.
https://doi.org/10.4040/jkan.20228
Impact of Obesity on Urinary Tract Infections in Korean Adults: Secondary Data Analysis Using Community-Based Cohort Study
Abstract
Purpose
This study investigated the incidence of urinary tract infection (UTI) in community-dwelling adults and identified the association between obesity and UTI.
Methods
The participants were 4,926 adults aged over 40 years who had no UTIs at the baseline survey of the Korean Genome Epidemiology Study. Obesity was defined according to the cirtieria of Korean Society for the Study of Obesity using body mass index (BMI) data. UTI was defined as those who had self-reported UTI or had either nitrite, or both leukocytes and blood in the urine dipstick test. Hazard ratio (HR) and 95% confidence interval (CI) were calculated using a multivariate Cox proportional hazards regression analysis to identify the association between the obesity and UTI.
Results
The incidence proportion of UTI was 5.1%, and the incidence density per 1,000 person-years was 25.5. After controlling general characteristics, people with BMI ≥30.0 kg/m2 remained 1.66 times (HR = 1.66, 95% CI = 1.06~2.60; p < .05) more likely to have UTI than those with normal weight. This trend was also present in men or people aged ≥ 60 years. Among women aged ≥ 60 years, people with BMI ≥ 30.0 kg/m2 were 1.98 times (HR = 1.98, 95% CI = 1.01~3.86; p < .05) more likely to have UTI than those with normal weight.
Conclusion
The BMI ≥ 30.0 kg/m2 is a risk factor of UTIs in Korean adult men over 40 years and women aged ≥ 60 years. It is necessary to emphasize the importance of obesity management to men or women aged ≥ 60 years, specifically.
INTRODUCTION
Obesity is one of the prevalent and serious health problems, both globally and domestically. Globally, the age-standardized prevalence of obesity with a body mass index (BMI) of more than 30.0 kg/m2 increased from 3.2% in 1975 to 10.8% in 2014 in men, and from 6.4% to 14.9% in women, and has continually increased in most other countries [1]. In 2015, excess weight contributed to 7.2% of all-cause deaths and 4.9% of all-cause disability-adjusted life years among adults globally. In Korea, the nationwide prevalence of obesity (BMI ≥ 25.0 kg/m2) over 30 years was 44.7% for men and 28.3% for women in 2018 [2], and BMI ≥ 30.0 kg/m2 was 2.81% for men and 3.11% for women [3]. According to the Korean National Claims Database, the incremental medical expenditure ratios based on people with normal weight was 38.4%, and 77.1% for people with BMI 30.0~34.9 kg/m2 and BMI ≥ 35.0 kg/m2, respectively, and people with BMI ≥ 35.0 kg/m2, men spent 1.15 times more and women spent 1.28 times more compared to those of normal weight [4].
Obesity is a well-known risk factor of certain cancers, cardiovascular diseases, and musculoskeletal disorders [3]. In addition, obesity has been shown to be associated with some infections [5]. Based on serological data analyses, a meta-analysis involving 5,739 subjects confirmed the association of Adenovirus 36 infection with obesity [6]. In a prospective Danish National Birth Cohort study, obesity in women (BMI ≥ 30.0 kg/m2) had a greater association with increased risk of community-acquired infections, including respiratory tract and skin and subcutaneous tissue infections, compared to women with normal weight [7]. A recent prospective study involving Swedish adults has also shown increased incidence of any infections, including skin and gastrointestinal tract infections in both obese men and women, and sepsis in obese women only, compared with normal weight subjects [8].
Although the mechanism underlying the association between obesity and infectious diseases is not well established, several possible mechanisms are proposed. First, chronic inflammation is common to obesity, and an expansion in fat cells promotes persistent stress and inflammation within adipose tissue, which can lead to fat cell apoptosis and prevent adequate production of anti-inflammatory mediators such as adiponectin [9, 10]. Second, obesity increases fat deposition in tissues of the immune organs such as the bone marrow and thymus, which lead to alterations in the distribution and activity of immune cells and overall immune defenses. The structural integrity of immune tissue is critical to proper production and maturation of immune cells, and any alteration in tissue integrity may reduce immune activity [10, 11]. Third, according to several animal models, diet-induced obesity alters innate and adaptive immune systems including cytokine change and decrease in immune cells including natural killer cells, dendritic cells, macrophages, and memory T cells [12, 13]. This alteration in the host's immune system promotes an environment likely to increase the risk of infection.
Urinary tract infection (UTI) is an infectious disease in the urinary system [14]. Community-acquired cystitis, one of the UTIs in Korea that has increased by 11.6% over five years from about 1.43 million cases in 2010 to 1.60 million cases in 2015 [15]. In 2019, 1.65 million patients visited outpatient clinics and spent $93 million on cystitis, ranking 40th among the most frequent outpatient diseases [16]. UTI that is not treated properly can lead to complications, such as hypertension, renal failure, and sepsis [17]. In Korea, disseminated osteomyelitis [18] and recurrent Guillain-Barré Syndrome [19] after UTI have been reported. In addition, antibiotic-resistant, particularly multidrug-resistant pathogens to UTI are a major concern and a public threat in Korea. Escherichia coli, Klebsiella pneumoniae, and Enterococcus species were the most prevalent pathogens observed in UTI, and resistance to commonly prescribed antibiotics has increased last 10 years [20, 21]. As UTIs acquired in a community setting requiring hospital admission or treatment in outpatient clinics have increased over time and are associated with complications, with a growing medical expenditure [8], the prevention of UTI through control of risk factors is important.
According to the literature review of the association between obesity and UTI, the findings are not consistent. Some studies have shown that obesity was associated with UTI in both men and women. In the analysis of people diagnosed with UTI over 5 years (2002~2006) using the U.S. private claims database, UTI was found to be significantly increased in obese men and women (BMI ≥ 30.0 kg/m2) [22]. In a cohort study using the Israeli non-for-profit insurance database in Israel, obesity (BMI ≥ 30.0 kg/m2) was identified as an independent risk factor for UTI in men and women, compared to subjects with BMI < 25.0 kg/m2 [23]. In a case-control study in Israel, the risk of recurrent UTI increased by four times for obese women (BMI > 30.0 kg/m2) [24]. On the contrary, in a prospective Danish National Birth Cohort study, obesity (BMI ≥ 30.0 kg/m2) was not associated with an increase in UTI in women [7]. In both a study with Swedish adults [8] and a prospective Danish Blood Donor study [25], obesity was associated with an increase in UTI in women but not in men. In a study in Iran, the BMI was not related to UTI when comparing BMI between adult patients diagnosed with UTI and healthy adults who underwent medical checkups [26].
Moreover, most previous studies on the relationship between obesity and UTI were conducted in Western countries, and few studies have reported the relationship between obesity and UTI in Korea. Considering that the obesity criteria are different between Western countries and Korea [27], it is necessary to identify the relationship between obesity and UTI in Koreans, and this result can be used as a strategy to prevent UTI. Therefore, this study investigated the incidence of UTI according to BMI group, and identified the association between obesity and UTI through a community-based cohort study data.
METHODS
1. Design and study participants
We conducted a secondary analysis of the data from the Ansan and Ansung cohort of the Korean Genome Epidemiology Study (KoGES) conducted by the Korea Disease Control and Prevention Agency (KDCA). The KoGES was a community-based prospective cohort study, and details of KoGES have previously been described [28]. The Ansan and Ansung cohort, representing small/medium-sized cities and rural areas in Korea, comprised 10,030 adult men and women aged between 40 and 69 years selected through two-stage cluster sampling based on information from the governing district in the telephone directory and information on demographic characteristics from the 2000 Census. The baseline survey of the cohort was completed in 2001 and 2002, and follow-up surveys are ongoing every other year. The cohort consists of a health examination and health interview of demographic information, medical history, family history of diseases, and health behaviors (including smoking, alcohol drinking, physical activity, and dietary intake).
Considering that obesity status changes over time and UTI can recur, we investigated the relationship between obesity and UTI occurrence during a possible short-term time course. As the most recent data available among the Ansan and Ansung cohort of KoGES was the 6th follow-up data, we used the 5th follow-up data as the baseline and identified the UTI incidence until 6th follow-up during a 2-year follow-up period. The 5th follow-up survey was conducted in 2011 and 2012 with 6,238 people, and the 6th was completed in 2013 and 2014 with 5,906 people [29].
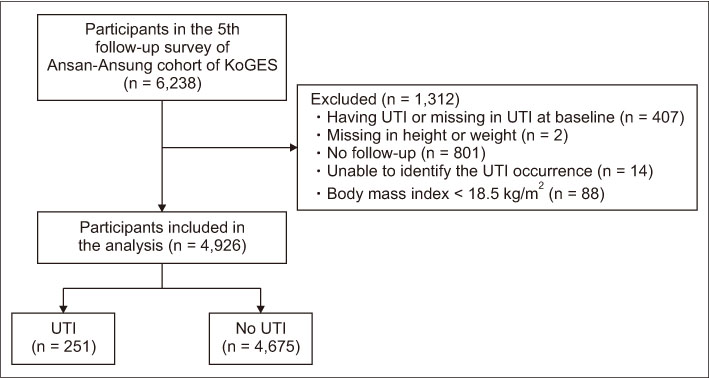
The inclusion criteria were participants who had no UTIs and no missing values in height or weight in the baseline (the 5th follow-up survey), no follow-up loss, no missing values in UTI incidence data during the follow-up period, and BMI ≥ 18.5 kg/m2. People with BMI < 18.5 kg/m2 were excluded due to small sample size (n = 88). Among the 6,238 people participated in the 5th follow-up survey, 1,312 who did not meet the inclusion criteria were excluded and the remaining 4,926 (response rate, 79.0%) were included in the analysis. Finally, UTI occurred in 251 (5.1%) during the two-year follow-up period (Figure 1).
Figure 1
Flowchart of study subjects' selection.
KoGES = Korean Genome Epidemiology Study; UTI = Urinary tract infection.
2. Study variables
The variables included in this study were obesity (independent variable), UTI (outcome variable), and general characteristics (covariates).
1) Obesity
Obesity was calculated and classified using BMI data, which utilized survey data on height and weight of the body measurement and calculated using the formula of weight (kg)/height (m)2. According to the classification of the Korean Society for the Study of Obesity [27], BMI is categorized into six groups, including “BMI < 18.5 kg/m2 (underweight),” “BMI 18.5~22.9 kg/m2 (normal weight),” “BMI 23.0~24.9 kg/m2 (pre-obese),” “BMI 25.0~29.9 kg/m2 (obese class I)”, “BMI 30.0~34.9 kg/m2 (obese class II),” and “BMI ≥ 35.0 kg/m2 (obese class III).” However, due to the small sample size of obese class III group at the baseline, we incorporated the obese class II and III into “obese class II+” (BMI ≥ 30.0 kg/m2).
2) Urinary tract infection
UTI was defined as those who had at least one of the two criteria: (1) self-reported UTI, (2) positive in either nitrite, or both leukocytes and blood based on the urine dipstick test [30]. The self-reported UTI was identified as newly occurred UTI based on the question, “Have you been diagnosed with a UTI by a doctor at a hospital or clinic?” during a 2-year follow-up period from the 5th (2011~2012) to 6th (2013~2014) follow-up survey, and categorized into “yes” or “no.” The diagnostic accuracy of urine dipstick test when both urine culture and UTI sign and symptom are considered a gold standard test for UTI. We used urine test data on “nitrite,” “white blood cell (WBC),” and “blood” and re-categorized according to the previous study [30]. For nitrite and blood in the urine, “1 positive” or greater, and “trace” or greater was classified as “positive.” For WBC in the urine, “1–3” or more were classified as “positive.” Among 251 UTI cases, only three were self-reported (1.2%), 238 were positive only in urine dipstick test (94.8%), and 10 were both (4.0%). Among 251 UTI cases, only three were self-reported (1.2%), 238 were positive only in urine dipstick test (94.8%), and 10 were both (4.0%).
3) General characteristics
The general characteristics consisted of factors known to be associated with UTI or infection in previous studies, including age [31, 32], gender [15, 33, 34], sexual behavior [31, 32], drinking [34], smoking [35], history of diabetes [31, 32, 36], and residence. Since the sexual behavior was not available from the KoGES database, currently living with spouse as a proxy of sexual behavior was measured and categorized as “yes” when married without divorce, separation or bereavement based on response to the survey question on marriage status, otherwise “no”. Drinking and smoking were categorized as “never,” “former,” and “current” based on response to the survey question on drinking or smoking status, and re-categorized into two groups of “currently drinking” and “currently smoking” or not, respectively. History of diabetes was categorized as “yes,” “no,” or “unknown” according to the response to the survey question on diabetes, “Have you ever been diagnosed the following diseases (e.g., diabetes) by a doctor.” Residence was categorized into “rural (Ansung)” or “urban (Ansan)” according to the survey area.
3. Data analysis
Data were analyzed using the IBM SPSS Statistics for Windows ver. 23.0 (IBM Corp., Armonk, NY, USA). The significance level of the two-tailed statistical test was .05. The participants' baseline characteristics were analyzed with frequency and percentage for categorical data and mean and standard deviation for continuous data. The differences in the BMI groups were compared using the chi-squared test, analysis of variance, and Tukey's post hoc test. The incidence of UTI was calculated using incidence proportion and incidence density, which was calculated by dividing the number of UTIs by the total number of participants or per 1,000 person-years, respectively. We calculated individual person-years by obtaining elapsed days from the 5th to the 6th follow-up dates, and by converting the days into years. Individual person-years was added together to obtain total person-years. The probability of remaining UTI free was determined by the Kaplan-Meier method, and the BMI groups were compared using the log-rank test. The incidence density of UTI among BMI groups was compared by simple Cox proportional hazards regression analysis. A multivariate Cox proportional hazards regression analysis was performed to identify the association of obesity and UTI incidence after controlling general characteristics in the baseline (the 5th follow-up survey). Subgroup and interaction analyses were performed to identify the relationship between BMI groups and UTI incidence according to gender and age groups (aged less than 60 years vs. aged older than and equal to 60 years).
4. Ethical considerations
This study was conducted after receiving approval of exempt review from the Institutional Review Board (IRB) of Pusan National University Yangsan Hospital (IRB No. 05-2018-072). Our data came from the department in charge of KoGES in KDCA after submitting the IRB approval letter and study proposal to the Department, and all data were provided in anonymized form.
RESULTS
1. Baseline characteristics of the participants according to the BMI group
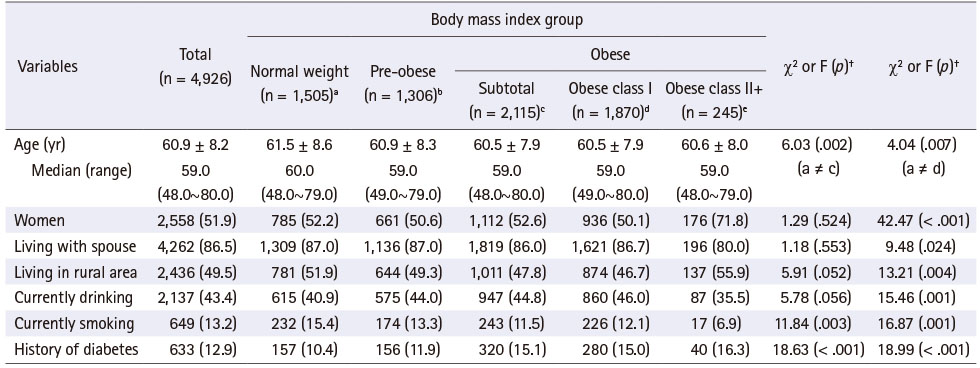
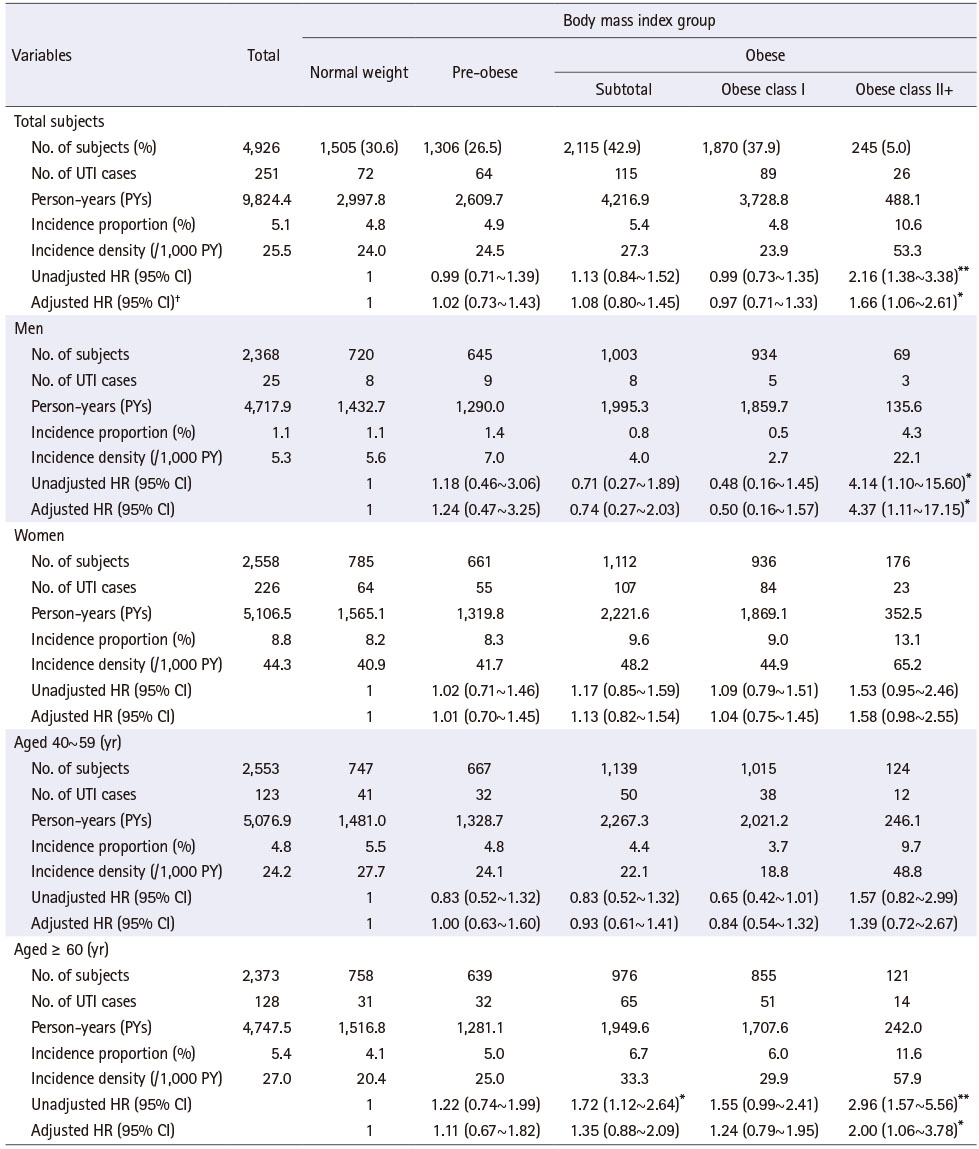
Among the 4,926 participants, for obese and obese class II+ people were 42.9% (n = 2,115) and 5.0% (n = 245), respectively. For total participants, the mean age was 60.9 years old, ranging from 48.0 to 80.0 years old, 13.2% were current smokers, and 12.9% had a history of diabetes. For obese and obese class II+ people, the mean age was 60.5 and 60.6 years old, current smokers were 11.5% and 6.9%, and with a history of diabetes were 15.1% and 16.3%, respectively. Obese people were younger, less likely to currently be smokers and had higher history of diabetes than the normal weight group. People in obese class II+ was higher in women and those living in a rural area, and lower in those living with a spouse, currently smokers, and currently drinkers than in the normal weight group (Table 1).
Table 1
Baseline Characteristics of Subjects according to the Body Mass Index Group
2. Incidence of urinary tract infection according to the BMI group
UTI incidence proportion was 4.8%, 5.4%, and 10.6%, and the incidence per 1,000 person-years was 24.0, 27.3, and 53.3 in the normal weight group, obese and obese class II+ people, respectively. UTI incidence in obese people was not significantly different from the normal weight group. People aged ≥ 60 years, obese people were 1.72 times more likely to have UTI than those with normal weight, but did not show an increase in risk after controlling general characteristics.
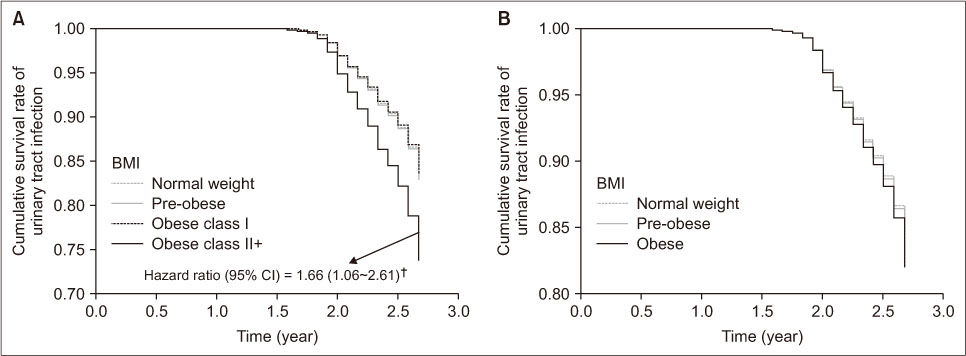
Contrary to obese people, UTI incidence was significantly higher in obese class II+ people than those with normal weight (p < .01). For the unadjusted hazard ratio (HR) of UTI incidence using normal weight as the reference group, people in obese class II+ were 2.16 times more likely to have UTI (p < .01). Kaplan-Meier survival analysis also revealed a significant relationship between BMI groups and UTI incidence, where people in obese class II+ had the lowest UTI free survival rate (log-rank p = .002). After controlling general characteristics, people in obese class II+ remained 1.66 times (HR = 1.66, 95% confidence interval [CI] = 1.06~2.61; p < .05) more likely to have UTI than those with normal weight (Figure 2). This trend was similar in men and people aged equal to and older than 60 years (aged ≥ 60 years). Men in obese class II+ were 4.37 times (HR = 4.37, 95% CI = 1.11~17.15; p < .05) more likely to have UTI than those with normal weight. For people aged ≥ 60 years or in obese class II+ increased the risk of developing UTI by 2.00 times (HR = 2.00, 95% CI = 1.06~3.78; p < .05) more than those with normal weight (Table 2).
Figure 2
Survival curves for urinary tract infection according to the body mass index (BMI) group after controlling the general characteristics. (A) Four groups of BMI; (B) three groups of BMI. †The reference of the hazard ratio was BMI of 18.5~22.9 kg/m2.
CI = Confidence interval.
Table 2
Incidence and Hazard Ratio of Urinary Tract Infection according to the Body Mass Index Group
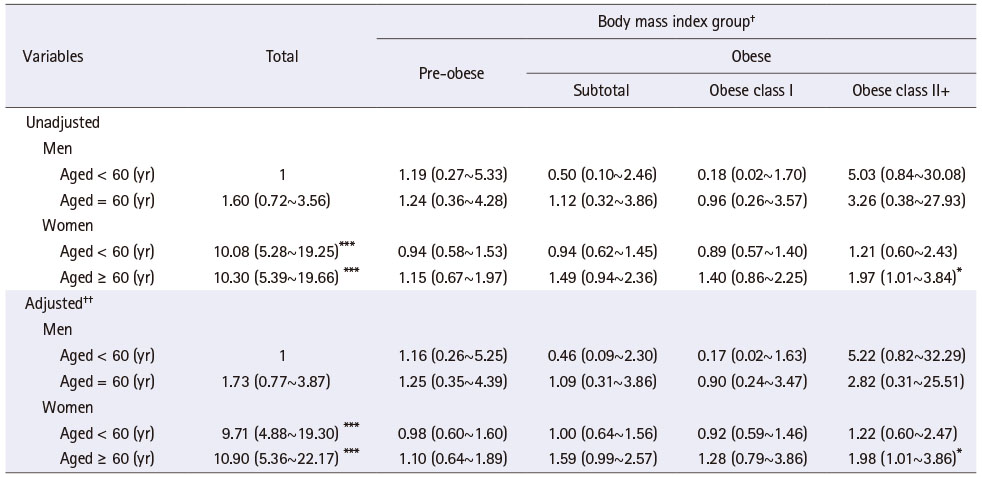
Table 3 showed the results of UTI risk according to BMI group by a combination of specific gender and age groups. UTI risk was 10.08 times and 10.30 times higher among women aged < 60 years and aged ≥ 60 years than men aged < 60 years, respectively, and the results were similar after controlling the general characteristics. Meanwhile, obese people did not show significant differences in UTI risk compared to those with normal weight in any combination of specific gender and age groups. However, women aged ≥ 60 years and in obese class II+ had 1.98 times (HR = 1.98, 95% CI = 1.01~3.86; p < 0.05) higher likelihood of having a UTI than those with normal weight, and the trend was the same after controlling the general characteristics.
Table 3
Hazard Ratio of Urinary Tract Infection according to Body Mass Index Group by Combination of Specific Gender and Age Groups
DISCUSSION
This study investigated the UTI incidence according to the BMI group and identified the association between obesity and UTI using a large-scale community-based cohort in Korea. UTI incidence in adults was very diverse in previous studies, ranging from 0.4% [25] to 9.4% [23] for men and 0.4% [25] to 22.7% [23], which may be related to differences in demographics and observation period. Comparing with the study conducted in Israel, which was similar in age, gender, and observation period of this study, the 5% of UTI incidence during the two-year follow-up in the current study was very low. UTI incidence was much higher in studies using insurance claims data [22, 23] than data from community residents [7, 8, 25]. Remarkably, the UTI incidence in the current study was much higher than in studies conducted in Denmark with a longer observation period [7, 25], which may be explained by the difference in age, a well-known risk factor of UTI [31, 32] and UTI definition. In the previously mentioned studies, UTI was defined according to the International Classification of Diseases (ICD), Ninth code [22, 23] or Tenth code [7, 8, 25], and there was almost no risk of recall bias by utilizing the cohort data or insurance claim data. However, we defined UTI cases as one of the two conditions: self-reported diagnosed UTI and urine dipstick test based on having nitrite or both leukocytes and blood [30]. Some people may forget their diagnosis since UTI is not a life-threatening condition, or others who had suspected signs and symptoms of UTI may not be diagnosed by visiting a hospital. Dipstick decision rule [30] was proven more sensitive and had higher negative predictive value than clinical decision rule based on having certain signs and symptoms; however, it still has a chance to lose the UTI cases due to relatively low negative predictive value (65.1%). About 96.0% of UTI cases in this study were identified by urine dipstick test. These limitations during UTI case findings can lead to underestimation of both UTI cases and incidence.
For the incidence of UTI among BMI groups, only obese people with BMI ≥ 30.0 kg/m2 were at higher risk of UTI than those with normal weight. This tendency continued in men after controlling general characteristics, which supports the previous study [23]. Unlike most previous studies [8, 23, 25], obesity was not associated with UTI incidence in women after controlling the general characteristics. However, this finding supports the findings of a study of Danish women using Danish National Birth Cohort (1996~2002) data [7]. Specifically, the Danish study revealed that the UTI risk was greater among underweight women than obese women [7]. However, as underweight women were excluded in the current study due to small sample size, further studies to identify the association between being underweight and UTI among Korean women are recommended. On the other hand, men with obese class II+ showed a higher UTI incidence than those of normal weight, which supports some previous studies that used insurance claims databases [22, 23]. This finding of the significant association between obesity and UTI only in men may be related to the small sample in men with obese class II+. That is, as the proportion of men with obese class II+ was also very small in a situation where the number of overall UTI cases was small in men, the UTI incidence in obese men may be overestimated. The association between obesity and UTI was found to be different according to age category based on average age of the participants (60 years old). That is, for women aged 60 years and over, UTI incidence was twice as high in people with obese class II+ than in those of normal weight. Older adults are more vulnerable to UTI than young people due to multiple factors, including an increase in urinary incontinence, retention, and catheterization, a decrease in immune function senescence, and comorbid conditions [31, 32].
1. Strengths and limitations of the study
This study has several strengths. First, few studies have attempted to identify the association between obesity and UTI incidence among adults in the community, and to the best of our knowledge, this is the first study in South Korea. Second, the KoGES, the data source for this study, adopted a prospective design to reduce the chance of recall bias and provided good evidence between obesity and the development of UTI. Moreover, it comprised a large sample size, which increased its statistical power. Third, the follow-up period was relatively short, reducing information bias due to changes in baseline characteristics, including BMI, and a large number were lost to follow-up over time. Fourth, as the weight and height data used in BMI calculations were not self-reported, but directly measured using a standardized method by trained surveyors, the accuracy of BMI can be guaranteed.
However, careful interpretation of the results is required due to the following limitations. First, as previously mentioned, UTI was defined by self-report and dipstick test results because urine culture data were not available in the KoGES, which may lead to underestimation of the UTI incidence with some errors. The dipstick decision rule has shown good interrater reliability [37] and better diagnostic precision than the clinical decision rule [30], but the limited negative predictive value still needs to be improved. Second, we could not use some variables associated with UTI development, such as the history of UTI and urinary system diseases, for adjustment. Sexual life is one of the known risk factors of UTI. Subjects were considered sexually active if they have a spouse, which may be different from their actual sexual life. Third, since we used the 5th follow-up data as a baseline, most participants were aged between 50s and 70s, reducing the generalizability of the findings to those aged less than 50 years. Moreover, this age mix may lead to bias in UTI incidence. For example, women in post-menopause are more vulnerable to UTI because the decrease in estrogen leads to change in urogenital epithelium and distribution of Lactobacillus, which facilitates the increase of uropathogens and infection [38]. Therefore, further studies involving people in their 30s and 40s are suggested. Lastly, since the Ansan and Ansung cohort of KoGES is not a nationally representative sample, generalizability of the findings to the entire population in South Korea may be limited.
2. Implications for clinical practice
This study provides evidence that obesity with BMI ≥ 30.0 kg/m2 is associated with development of UTI. Particularly, obesity in men over 40 years or women aged ≥ 60 years was found to be more strongly related to the development of UTIs in this study. Men may be less interested in UTI because of lower UTI incidence than women; however, UTI risk is rapidly increasing among men with obese class II+. By understanding the association between obesity and UTI, nurses in community settings should identify these high-risk groups for UTI and utilize the findings in health education for the public. In addition, policymakers should understand the additional benefits of weight loss and make efforts to manage obesity.
CONCLUSION
This study analyzed the association between obesity and UTI among Korean adults in the community. The UTI incidence in Korean adults is increasing only in obese people with BMI ≥ 30.0 kg/m2, particularly, among men regardless of age, or women aged ≥ 60 years. It seems that obesity with BMI ≥ 30.0 kg/m2 can be a moderator of UTI. Nurses should utilize the findings in health education for the public and emphasize the importance of obesity control to men or women aged ≥ 60 years specifically.
This manuscript is a revision of the first author's master's thesis from Pusan National University. Year of 2019.
This work was presented at Korean Society of Biological Nursing Science Conference, 12, 2019, Seoul, Republic of Korea.
CONFLICTS OF INTEREST:The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS:
Conceptualization or/and Methodology: Seo SH & Jeong IS.
Data curation or/and Analysis: Seo SH & Jeong IS.
Funding acquisition: None.
Investigation: Seo SH & Jeong IS.
Project administration or/and Supervision: Jeong IS & Lee EJ.
Resources or/and Software: None.
Validation: Jeong IS & Lee EJ.
Visualization: Jeong IS & Lee EJ.
Writing original draft or/and Review & Editing: Seo SH & Jeong IS & Lee EJ.
ACKNOWLEDGEMENTS
None.
DATA SHARING STATEMENT
Please contact the corresponding author for data availability.
References
-
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387(10026):1377–1396. [doi: 10.1016/S0140-6736(16)30054-X]
-
-
Ministry of Health & Welfare (MOHW). Korea Disease Control and Prevention Agency (KDCA). Korea health statistics 2018: Korea National Health and Nutrition Examination Survey (KNHANES VII-3). Cheongju: KDCA; 2019 Dec.Report No.: 11-1351159-000027-10.
-
-
GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. The New England Journal of Medicine 2017;377(1):13–27. [doi: 10.1056/NEJMoa1614362]
-
-
Harpsøe MC, Nielsen NM, Friis-Møller N, Andersson M, Wohlfahrt J, Linneberg A, et al. Body mass index and risk of infections among women in the Danish National Birth Cohort. American Journal of Epidemiology 2016;183(11):1008–1017. [doi: 10.1093/aje/kwv300]
-
-
Ghilotti F, Bellocco R, Ye W, Adami HO, Trolle Lagerros Y. Obesity and risk of infections: Results from men and women in the Swedish National March Cohort. International Journal of Epidemiology 2019;48(6):1783–1794. [doi: 10.1093/ije/dyz129]
-
-
Tagliabue C, Principi N, Giavoli C, Esposito S. Obesity: Impact of infections and response to vaccines. European Journal of Clinical Microbiology & Infectious Diseases 2016;35(3):325–331. [doi: 10.1007/s10096-015-2558-8]
-
-
Torres L, Martins VD, Faria AMC, Maioli TU. The intriguing relationship between obesity and infection. Journal of Infectiology 2018;1(1):6–10.
-
-
Wie SH. Diagnosis and treatment of urinary tract infections; Proceedings of The Korean Journal of Medicine, Annual spring conference 2014; 2014 Apr 1; Seoul. Seoul: The Korean Association of Internal Medicine; c2014. pp. 22-26.
-
-
National Health Insurance Service. Cystitis, it is the most common in the 50's women [internet]. Wonju: National Health Insurance Service; c2016 [cited 2020 Aug 20].
-
-
Health Insurance Review and Assessment Service (HIRA). 2019 Medical expenditure review results [Internet]. Wonju: HIRA; c2020 [cited 2020 Aug 20].Available from: http://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020045030000&brdScnBltNo=4&brdBltNo=2405&pageIndex=1#-
none.
-
-
Okubo Y, Handa A. The impact of obesity on pediatric inpatients with urinary tract infections in the United States. Journal of Pediatric Urology 2017;13(5):455.e1–455.e5. [doi: 10.1016/j.jpurol.2017.03.038]
-
-
Lee JH, Subhadra B, Son YJ, Kim DH, Park HS, Kim JM, et al. Phylogenetic group distributions, virulence factors and antimicrobial resistance properties of uropathogenic Escherichia coli strains isolated from patients with urinary tract infections in South Korea. Letters in Applied Microbiology 2016;62(1):84–90. [doi: 10.1111/lam.12517]
-
-
Shin HR, Moon J, Lee HS, Ahn SJ, Kim TJ, Jun JS, et al. Increasing prevalence of antimicrobial resistance in urinary tract infections of neurological patients, Seoul, South Korea, 2007-2016. International Journal of Infectious Diseases 2019;84:109–115. [doi: 10.1016/j.ijid.2019.05.002]
-
-
Seo MH, Lee WY, Kim SS, Kang JH, Kang JH, Kim KK, et al. 2018 Korean Society for the Study of Obesity guideline for the management of obesity in Korea. Journal of Obesity & Metabolic Syndrome 2019;28(1):40–45. [doi: 10.7570/jomes.2019.28.1.40]
-
-
Korea Disease Control and Prevention Agency (KDCA). Status by cohort [Internet]. Cheongju: KDCA; c2020 [cited 2020 Aug 20].Available from: http://www.kdca.go.kr/contents.es?mid=a40504030900.
-
-
Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al. Dipsticks and diagnostic algorithms in urinary tract infection: Development and validation, randomised trial, economic analysis, observational cohort and qualitative study. Health Technology Assessment 2009;13(19):iii–iv. ix–xi, 1–73. [doi: 10.3310/hta13190]
-
-
Mama M, Manilal A, Gezmu T, Kidanewold A, Gosa F, Gebresilasie A. Prevalence and associated factors of urinary tract infections among diabetic patients in Arba Minch Hospital, Arba Minch province, South Ethiopia. Turkish Journal of Urology 2018;45(1):56–62. [doi: 10.5152/tud.2018.32855]
-
-
Aydın A, Kocaöz S, Kara P. Prevalence of lower urinary tract symptoms in pregnant adolescents and the influencing factors. Journal of Pediatric and Adolescent Gynecology 2020;33(2):160–166. [doi: 10.1016/j.jpag.2019.10.007]
-
-
Duanngai K, Sirasaporn P, Ngaosinchai SS. The reliability and validity of using the urine dipstick test by patient self-assessment for urinary tract infection screening in spinal cord injury patients. Journal of Family Medicine and Primary Care 2017;6(3):578–582. [doi: 10.4103/2249-4863.222024]
-
- Related articles
 KSNS
KSNS
 E-SUBMISSION
E-SUBMISSION

 Cite
Cite