Articles
- Page Path
- HOME > J Korean Acad Nurs > Volume 55(2); 2025 > Article
-
Research Paper
- A non-face-to-face diabetes self-management program based on self-efficacy theory and health literacy: a non-randomized controlled trial
-
Jung Hee Lee
 , Soo Jin Lee
, Soo Jin Lee
-
Journal of Korean Academy of Nursing 2025;55(2):165-177.
DOI: https://doi.org/10.4040/jkan.25009
Published online: May 23, 2025
Department of Nursing, Korea National Open University, Seoul, Korea
- Corresponding author: Soo Jin Lee Department of Nursing, Korea National Open University, 86 Daehak-ro, Jongno-gu, Seoul 03087, Korea E-mail: syjlee@knou.ac.kr
- *This manuscript is a revision of the first author’s master’s thesis from Korea National Open University (2022).
© 2025 Korean Society of Nursing Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution NoDerivs License (http://creativecommons.org/licenses/by-nd/4.0) If the original work is properly cited and retained without any modification or reproduction, it can be used and re-distributed in any format and medium.
- 3,339 Views
- 243 Download
Abstract
-
Purpose
- This study aimed to assess the impact of a non-face-to-face diabetes self-management program based on self-efficacy theory and focusing on health literacy.
-
Methods
- A quasi-experimental, nonequivalent control group pre–post design was used. Participants from a community health promotion center were included if they (1) were 30–70 years of age, (2) had type 2 diabetes with glycated hemoglobin (HbA1c) ≥6.5%, and (3) had internet access via computers or mobile devices. The 8-week program was developed based on self-efficacy theory, and it included virtual education using an online platform, telephone counseling, videos, and social networking site activities considering health literacy. Fasting blood glucose levels, HbA1c levels, diabetes self-efficacy, social support, depression, and self-management behaviors were assessed. Data were analyzed using the independent t-test, paired t-test, and others.
-
Results
- Post-test results showed that the intervention group had significantly lower fasting blood glucose levels and improved diabetes self-efficacy, social support, and self-management behaviors compared with the control group. An analysis of the pre-to-post changes in scores indicated that the intervention group had significantly greater improvements in fasting blood glucose levels, diabetes self-efficacy, and overall diabetes self-management behaviors than those observed in the control group.
-
Conclusion
- Non-face-to-face programs based on self-efficacy theory that consider health literacy can provide effective diabetes management support to patients when in-person diabetes management at community health centers is challenging.
Introduction
Methods
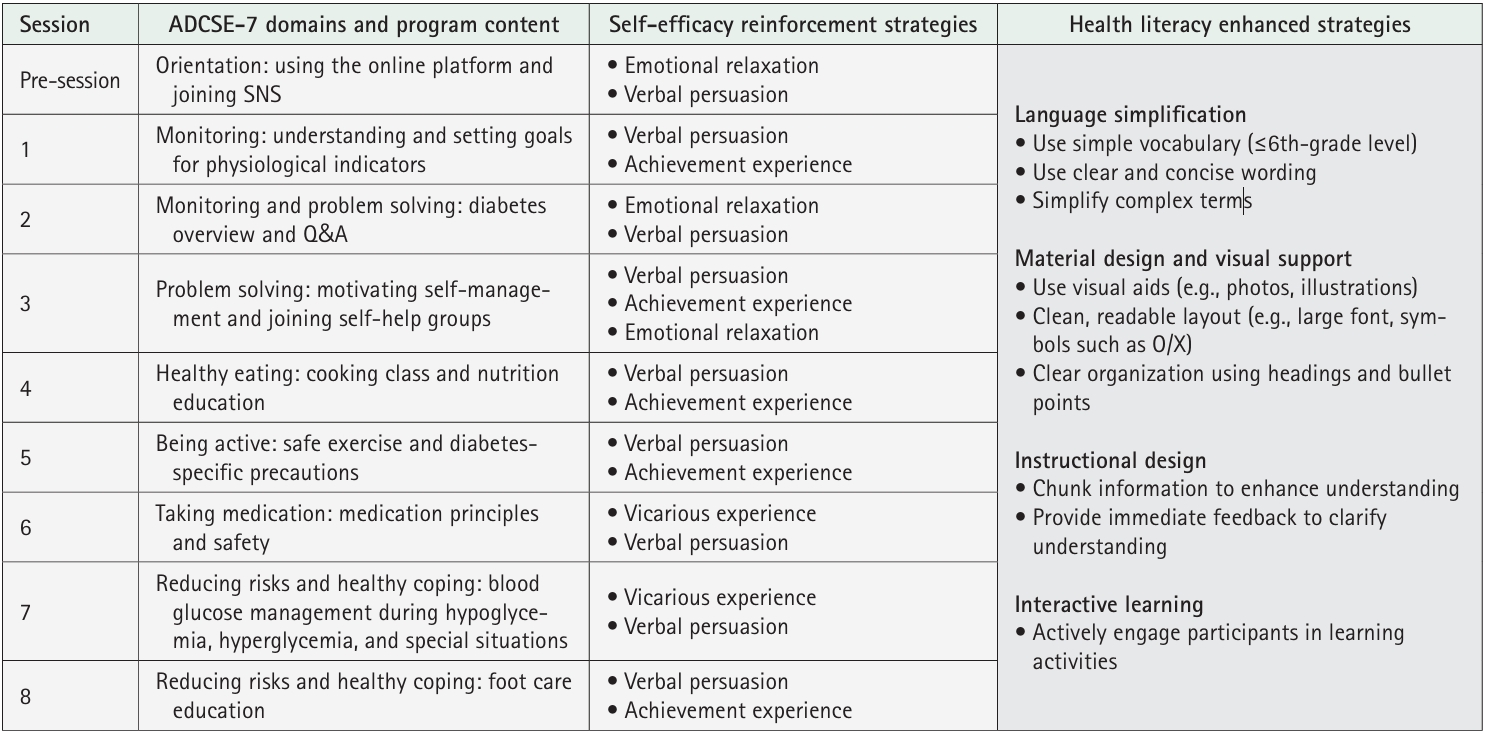
1) Virtual education
2) Educational booklets
3) Telephone counseling
4) Educational videos
5) Social networking site activities
1) Diabetes-related physiological outcomes
2) Diabetes-related psychosocial outcomes
3) Diabetes self-management behaviors
Results
Discussion
Conclusion
-
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
-
Acknowledgements
The authors sincerely thank the participants for their generous involvement in the program, especially during the challenges posed by the COVID-19 pandemic. We also appreciate the dedication and support of the staff involved in conducting the study at the community health promotion center in Busan, South Korea.
-
Funding
This research received no external funding.
-
Data Sharing Statement
Please contact the corresponding author for data availability.
-
Author Contributions
Conceptualization and Methodology: JHL, SJL. Data curation or/and Analysis: JHL, SJL. Funding acquisition: none. Investigation: JHL. Project administration or/and Supervision: SJL. Resources or/and Software: JHL. Validation: SJL. Visualization: SJL. Writing original draft or/and Review & Editing: JHL, SJL. Final approval of the manuscript: all authors.
Article Information
| Characteristic | Intervention group (n=27) | Control group (n=24) | t or χ2 or U | p |
|---|---|---|---|---|
| Age (yr) | 0.27 | .869 | ||
| <60 | 13 (48.1) | 11 (45.8) | ||
| ≥60 | 14 (51.9) | 13 (54.2) | ||
| Sex | 0.32 | .570 | ||
| Men | 6 (22.2) | 7 (29.2) | ||
| Women | 21 (77.8) | 17 (70.8) | ||
| Education | 1.79 | .408 | ||
| ≤Middle school | 9 (33.3) | 8 (33.3) | ||
| High school | 14 (51.9) | 9 (37.5) | ||
| College or more | 4 (14.8) | 7 (29.2) | ||
| Duration of diabetes (yr) | 0.77 | .802a) | ||
| 1–5 | 15 (55.6) | 14 (58.3) | ||
| 6–10 | 7 (25.9) | 4 (16.7) | ||
| ≥11 | 5 (18.5) | 6 (25.0) | ||
| Comorbidities | 0.02 | >.999 | ||
| Yes | 25 (92.6) | 22 (91.7) | ||
| No | 2 (7.4) | 2 (8.3) | ||
| Medication usage | 0.32 | .736a) | ||
| Yes | 22 (81.5) | 18 (75.0) | ||
| No | 5 (18.5) | 6 (25.0) | ||
| Experience in diabetes education | 1.85 | .255a) | ||
| Yes | 6 (22.2) | 2(8.3) | ||
| No | 21 (77.8) | 22 (91.7) | ||
| Smoking | 0.11 | >.999 | ||
| Yes | 3 (11.1) | 2 (8.3) | ||
| No | 24 (88.9) | 22 (91.7) | ||
| Drinking | 0.91 | .451a) | ||
| Yes | 3 (11.1) | 5 (20.8) | ||
| No | 24 (88.9) | 19 (79.2) | ||
| FBG (mg/dL) | 129.63±29.12 | 128.29±25.78 | 321.50 | .966b) |
| A1C level (%) | 6.78±0.96 | 6.74±0.84 | 317.00 | .899b) |
| SBP (mm Hg) | 126.48±13.01 | 123.54±12.02 | 0.83 | .408 |
| DBP (mm Hg) | 76.15±9.08 | 76.96±8.65 | –0.33 | .746 |
| Total cholesterol (mg/dL) | 158.81±40.99 | 170.46±41.95 | 252.00 | .177b) |
| HDL cholesterol (mg/dL) | 42.93±14.95 | 47.71±15.11 | –1.16 | .253 |
| Triglycerides (mg/dL) | 157.07±73.78 | 159.29±80.45 | 322.00 | .974b) |
| LDL cholesterol (mg/dL) | 84.70±30.48 | 91.50±34.25 | –0.75 | .457 |
| BMI (kg/m2) | 25.89±5.96 | 24.52±2.71 | 311.50 | .819b) |
| Diabetes self-efficacy | 44.85±5.91 | 42.54±6.45 | 223.00 | .056b) |
| Diabetes social support | 16.22±7.52 | 15.00±6.99 | 293.00 | .563b) |
| Depression | 13.44±4.71 | 16.63±6.47 | 235.50 | .094b) |
| DSMB | 72.14±16.89 | 69.79±16.78 | 0.50 | .620 |
| Diet | 19.11±8.28 | 20.58±6.34 | –0.71 | .483 |
| Exercise | 7.78±3.00 | 8.33±3.60 | 294.50 | .578b) |
| Blood glucose monitoring | 6.63±5.41 | 4.13±3.68 | 225.00 | .059b) |
| Medications | 15.48±5.66 | 13.54±6.09 | 256.00 | .183b) |
| Foot care | 23.19±6.98 | 23.21±7.86 | –0.01 | .991 |
Values are presented as number (%) or mean±standard deviation.
HbA1c, glycated hemoglobin; BMI, body mass index; DBP, diastolic blood pressure; DSMB, diabetes self-management behavior; FBG, fasting blood glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
a)By Fisher’s exact test.
b)By Mann-Whitney U test.
| Variable | Intervention group (n=27) | Control group (n=24) | t or U | p |
|---|---|---|---|---|
| FBG (mg/dL) | 113.85±12.91 | 126.75±25.67 | –2.22 | .033 |
| HbA1c level (%) | 6.42±0.75 | 6.39±0.84 | 0.19 | .848 |
| SBP (mm Hg) | 119.33±9.75 | 119.79±11.13 | –0.16 | .877 |
| DBP (mm Hg) | 74.04±8.06 | 73.79±7.51 | 0.11 | .911 |
| Total cholesterol (mg/dL) | 165.00±36.78 | 169.21±47.12 | 322.50 | .491a) |
| HDL cholesterol (mg/dL) | 49.48±17.59 | 49.63±14.40 | –0.03 | .975 |
| Triglycerides (mg/dL) | 142.37±80.80 | 143.92±78.06 | 322.50 | .491a) |
| LDL cholesterol (mg/dL) | 86.11±30.01 | 93.29±43.89 | 310.50 | .804a) |
| BMI (kg/m2) | 25.48±5.98 | 23.73±2.77 | 1.37 | .179 |
| Diabetes self-efficacy | 57.40±5.15 | 51.04±5.69 | 4.19 | <.001 |
| Diabetes social support | 26.19±2.43 | 21.75±4.67 | 4.32 | <.001 |
| Depression | 11.04±1.99 | 14.17±5.19 | 223.50 | .055a) |
| DSMB | 89.74±10.57 | 77.96±11.58 | 3.80 | <.001 |
| Diet | 25.67±5.73 | 23.04±5.03 | 1.73 | .090 |
| Exercise | 11.11±2.79 | 9.29±3.48 | 2.07 | .044 |
| Blood glucose monitoring | 11.52±3.49 | 6.13±3.69 | 5.38 | <.001 |
| Medications | 16.11±4.37 | 13.92±5.76 | 267.50 | .184a) |
| Foot care | 25.33±6.54 | 25.58±7.25 | –0.13 | .897 |
Values are presented as mean±standard deviation.
HbA1c, glycated hemoglobin; BMI, body mass index; DBP, diastolic blood pressure; DSMB, diabetes self-management behavior; FBG, fasting blood glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
a)By Mann-Whitney U test.
| Variable | Group | Pre-test | Post-test | t or Z | p | Change (post-pre) | t or U | p |
|---|---|---|---|---|---|---|---|---|
| FBG (mg/dL) | I | 129.63±29.12 | 113.85±12.91 | –3.28 | <.001a) | –15.78±25.69 | 192.00 | .012b) |
| C | 128.29±25.78 | 126.75±25.67 | 0.79 | .439 | –1.54±9.60 | |||
| HbA1c level (%) | I | 6.78±0.96 | 6.42±0.75 | –2.85 | .002a) | –0.36±0.61 | 299.00 | .649b) |
| C | 6.74±0.84 | 6.39±0.84 | 4.09 | <.001 | –0.37±0.44 | |||
| SBP (mm Hg) | I | 126.48±13.01 | 119.33±9.75 | 2.44 | .022 | –7.15±15.25 | –0.82 | .419 |
| C | 123.54±12.02 | 119.79±11.13 | 1.28 | .214 | –3.75±14.38 | |||
| DBP (mm Hg) | I | 76.15±9.08 | 74.04±8.06 | 1.08 | .289 | –2.11±10.12 | 0.37 | .715 |
| C | 76.96±8.65 | 73.79±7.51 | 1.49 | .149 | –3.17±10.40 | |||
| Total cholesterol (mg/dL) | I | 158.81±40.99 | 165.00±36.78 | –1.57 | .118a) | 6.19±36.00 | 256.00 | .203b) |
| C | 170.46±41.95 | 169.21±47.12 | 0.17 | .871 | –1.25±37.19 | |||
| HDL cholesterol (mg/dL) | I | 42.93±14.95 | 49.48±17.59 | –2.69 | .012 | 6.56±12.67 | 1.27 | .211 |
| C | 47.71±15.11 | 49.63±14.40 | –0.70 | .492 | 1.92±13.44 | |||
| Triglycerides (mg/dL) | I | 157.07±73.78 | 142.37±80.80 | 0.97 | .342 | –14.7±78.86 | 0.03 | .974 |
| C | 159.29±80.45 | 143.92±78.06 | 1.15 | .262 | –15.38±65.55 | |||
| LDL cholesterol (mg/dL) | I | 84.70±30.48 | 86.11±30.01 | –0.27 | .790 | 1.41±27.13 | –0.05 | .960 |
| C | 91.50±34.25 | 93.29±43.89 | –0.32 | .753 | 1.79±27.59 | |||
| BMI (kg/m2) | I | 25.89±5.96 | 25.48±5.98 | –1.01 | .325a) | –0.41±6.07 | 292.00 | .552b) |
| C | 24.52±2.71 | 23.73±2.77 | –3.62 | <.001a) | –0.79±1.31 | |||
| Diabetes self-efficacy | I | 44.85±5.91 | 57.40±5.15 | –12.04 | <.001 | 12.56±5.42 | 184.50 | .008b) |
| C | 42.54±6.45 | 51.04±5.69 | –3.89 | <.001a) | 8.50±7.25 | |||
| Diabetes social support | I | 16.22±7.52 | 26.19±2.43 | –7.15 | <.001 | 9.96±7.25 | 1.74 | .089 |
| C | 15.00±6.99 | 21.75±4.67 | –5.73 | <.001 | 6.75±5.77 | |||
| Depression | I | 13.44±4.71 | 11.04±1.99 | –3.07 | .002a) | –2.41±3.75 | 311.00 | .810b) |
| C | 16.63±6.47 | 14.17±5.19 | –3.02 | .001a) | –2.46±3.34 | |||
| DSMB | I | 72.14±16.89 | 89.74±10.57 | –5.34 | <.001 | 17.59±17.13 | 2.14 | .037 |
| C | 69.79±16.78 | 77.96±11.58 | –2.89 | .008 | 8.17±13.86 | |||
| Diet | I | 19.11±8.28 | 25.67±5.73 | –5.40 | <.001 | 6.56±6.31 | 2.42 | .019 |
| C | 20.58±6.34 | 23.04±5.03 | –2.10 | .047 | 2.46±5.73 | |||
| Exercise | I | 7.78±3.00 | 11.11±2.79 | –6.09 | <.001 | 3.33±2.84 | 2.79 | .007 |
| C | 8.33±3.60 | 9.29±3.48 | –1.45 | .160 | 0.96±3.24 | |||
| Blood glucose monitoring | I | 6.63±5.41 | 11.52±3.49 | –4.41 | <.001 | 4.89±5.77 | 207.50 | .026b) |
| C | 4.13±3.68 | 6.13±3.69 | –2.41 | .014a) | 2.00±3.68 | |||
| Medications | I | 15.48±5.66 | 16.11±4.37 | –0.75 | .452a) | 0.63±4.23 | 287.00 | .461b) |
| C | 13.54±6.09 | 13.92±5.76 | –0.88 | .489a) | 0.38±3.03 | |||
| Foot care | I | 23.19±6.98 | 25.33±6.54 | –1.12 | .263a) | 2.15±8.20 | 321.00 | .959b) |
| C | 23.21±7.86 | 25.58±7.25 | –1.46 | .158 | 2.38±7.97 |
Values are presented as mean±standard deviation.
HbA1c, glycated hemoglobin; BMI, body mass index; C, control; DBP, diastolic blood pressure; DSMB, diabetes self-management behavior; FBG, fasting blood glucose; HDL, high-density lipoprotein; I, intervention; LDL, low-density lipoprotein; SBP, systolic blood pressure.
a)By Wilcoxon signed rank test.
b)By Mann-Whitney U test.
- 1. Statistics Korea. 2020 Cause of death statistics [Internet]. Statistics Korea; 2021 [cited 2025 Mar 2]. Available from: https://kostat.go.kr/board.es?mid=a10301060200&bid=218&act=view&list_no=403046
- 2. Korea Disease Control and Prevention Agency. 2020 National Health Statistics: 8th Korea National Health and Nutrition Examination Survey (KNHANES), 2nd year [Internet]. Korea Disease Control and Prevention Agency; 2022 [cited 2025 Mar 2]. Available from: https://knhanes.kdca.go.kr/knhanes/main.do
- 3. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865. https://doi.org/10.1016/S0140-6736(98)07037-8ArticlePubMed
- 4. Korea Disease Control and Prevention Agency. 2021 Community health statistics at a glance [Internet]. Korea Disease Control and Prevention Agency; 2022 [cited 2025 Mar 2]. Available from: https://chs.kdca.go.kr/chs/stats/statsMain.do
- 5. World Health Organization. Diabetes [Internet]. World Health Organization; 2024 [cited 2025 Mar 2]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
- 6. World Health Organization. Self-care for health and well-being [Internet]. World Health Organization; 2024 [cited 2025 Mar 2]. Available from: https://www.who.int/news-room/fact-sheets/detail/self-care-health-interventions
- 7. Powers MA, Bardsley JK, Cypress M, Funnell MM, Harms D, Hess-Fischl A, et al. Diabetes self-management education and support in adults with type 2 diabetes: a consensus report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Diabetes Care. 2020;43(7):1636-1649. https://doi.org/10.2337/dci20-0023ArticlePubMed
- 8. Korea Centers for Disease Control and Prevention. Chronic disease prevention and control: hypertension diabetes registry [Internet]. Korea Disease Control and Prevention Agency; 2019 [cited 2025 Mar 2]. Available from: https://kdca.go.kr/contents.es?mid=a20303020200
- 9. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191-215. https://doi.org/10.1037//0033-295x.84.2.191ArticlePubMed
- 10. Jiang X, Wang J, Lu Y, Jiang H, Li M. Self-efficacy-focused education in persons with diabetes: a systematic review and meta-analysis. Psychol Res Behav Manag. 2019;12:67-79. https://doi.org/10.2147/PRBM.S192571ArticlePubMedPMC
- 11. Park H, Hwang SK. Linguistic and functional health literacy among community-dwelling old adults. Glob Health Nurs. 2014;4(2):49-58. https://rins.pusan.ac.kr/sites/rins/pdf/4-2-1.pdf
- 12. Baker DW, Parker RM, Williams MV, Clark WS, Nurss J. The relationship of patient reading ability to self-reported health and use of health services. Am J Public Health. 1997;87(6):1027-1030. https://doi.org/10.2105/ajph.87.6.1027ArticlePubMedPMC
- 13. Baker DW, Wolf MS, Feinglass J, Thompson JA, Gazmararian JA, Huang J. Health literacy and mortality among elderly persons. Arch Intern Med. 2007;167(14):1503-1509. https://doi.org/10.1001/archinte.167.14.1503ArticlePubMed
- 14. Sudore RL, Mehta KM, Simonsick EM, Harris TB, Newman AB, Satterfield S, et al. Limited literacy in older people and disparities in health and healthcare access. J Am Geriatr Soc. 2006;54(5):770-776. https://doi.org/10.1111/j.1532-5415.2006.00691.xArticlePubMed
- 15. Butayeva J, Ratan ZA, Downie S, Hosseinzadeh H. The impact of health literacy interventions on glycemic control and self-management outcomes among type 2 diabetes mellitus: a systematic review. J Diabetes. 2023;15(9):724-735. https://doi.org/10.1111/1753-0407.13436ArticlePubMedPMC
- 16. Choi SA. Effects of distance memory training intervention on cognitive function, memory self-efficacy, and depression in older adults with subjective memory complaints [master's thesis]. Seoul: Korea National Open University; 2021.
- 17. Son HR, Park SY, Yong HJ, Ko YJ, Jung DW, Won ES, et al. YouTube self-management education for hypertensive patients in the COVID-19 pandemic era: is this non-face-to-face program satisfactory in a community? Korean J Health Educ Promot. 2021;38(5):85-101. https://doi.org/10.14367/kjhep.2021.38.5.85Article
- 18. World Health Organization. Implementing telemedicine services during COVID-19: guiding principles and considerations for a stepwise approach [Internet]. World Health Organization; 2020 [cited 2025 Mar 2]. Available from: https://www.who.int/publications/i/item/WPR-DSE-2020-032
- 19. Oh EG. Perspectives on nursing profession for a post-COVID-19 new normal. Korean J Adult Nurs. 2020;32(3):221-222. https://doi.org/10.7475/kjan.2020.32.3.221Article
- 20. Greenwood DA, Gee PM, Fatkin KJ, Peeples M. A systematic review of reviews evaluating technology-enabled diabetes self-management education and support. J Diabetes Sci Technol. 2017;11(5):1015-1027. https://doi.org/10.1177/1932296817713506ArticlePubMedPMC
- 21. Robson N, Hosseinzadeh H. Impact of telehealth care among adults living with type 2 diabetes in primary care: a systematic review and meta-analysis of randomised controlled trials. Int J Environ Res Public Health. 2021;18(22):12171. https://doi.org/10.3390/ijerph182212171ArticlePubMedPMC
- 22. Choi ES, Yeom EY. The effects of diabetes management using mobile application on physiological indicators and self-care behaviors of type 2 diabetes mellitus patients. J Wellness. 2019;14(3):401-411. https://doi.org/10.21097/ksw.2019.08.14.3.401Article
- 23. Song MS, Kim HS. Effects of diabetes education and telephone counseling on depression in patients with diabetes. J Korean Acad Adult Nurs. 2008;20(3):481-488.PDF
- 24. Korea Health Promotion Institute. 2021 Casebook of non-face-to-face implementation of community integrated health promotion projects [Internet]. Korea Health Promotion Institute; 2022 [cited 2025 Mar 2]. Available from: https://www.khealth.or.kr/kps/publish/list?menuId=MENU00890&page_no=B2017003
- 25. Korean Diabetes Association. 2021 Diabetes mellitus guidelines, 7th edition: clinical practice guidelines for diabetes. Korean Diabetes Association; 2021. 310 p.
- 26. Tshiananga JK, Kocher S, Weber C, Erny-Albrecht K, Berndt K, Neeser K. The effect of nurse-led diabetes self-management education on glycosylated hemoglobin and cardiovascular risk factors: a meta-analysis. Diabetes Educ. 2011;38(1):108-123. https://doi.org/10.1177/0145721711423978ArticlePubMed
- 27. Zare S, Ostovarfar J, Kaveh MH, Vali M. Effectiveness of theory-based diabetes self-care training interventions; a systematic review. Diabetes Metab Syndr. 2020;14(4):423-433. https://doi.org/10.1016/j.dsx.2020.04.008ArticlePubMed
- 28. Han S, Lee G. Comparative analysis of instructors’ perception of synchronous online classes: a case study of a university. Cult Converg. 2020;42(7):395-418. https://doi.org/10.33645/cnc.2020.07.42.7.395Article
- 29. Association of Diabetes Care and Education Specialists; Kolb L. An effective model of diabetes care and education: the ADCES7 Self-Care Behaviors(TM). Sci Diabetes Self Manag Care. 2021;47(1):30-53. https://doi.org/10.1177/0145721720978154ArticlePubMed
- 30. Korean Diabetes Association Education Committee. Diabetes education guidelines, 4th edition. Korean Diabetes Association; 2019. 363 p.
- 31. Ntiri DW, Stewart M. Transformative learning intervention: effect on functional health literacy and diabetes knowledge in older African Americans. Gerontol Geriatr Educ. 2009;30(2):100-113. https://doi.org/10.1080/02701960902911265ArticlePubMed
- 32. Korea Centers for Disease Control and Prevention. Social distancing in daily life: a new normal for overcoming COVID-19 [Internet]. Korea Centers for Disease Control and Prevention; 2020 [cited 2025 Mar 2]. Available from: https://www.kdca.go.kr/gallery.es?mid=a20503020000&bid=0003&act=view&list_no=144684
- 33. Song M, Choi S, Kim SA, Seo K, Lee SJ, Kim EH. Development and validation of the diabetes management self-efficacy scale for older adults (DMSES-O). J Muscle Jt Health. 2014;21(3):184-194. https://doi.org/10.5953/JMJH.2014.21.3.184Article
- 34. Byun SH. A structural modeling for quality of life with diabetes: associated with diabetes locus of control, social support, self-efficacy, and coping strategy [dissertation]. Gimhae: Inje University; 2016.
- 35. Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the Diabetes Care Profile. Eval Health Prof. 1996;19(2):208-230. https://doi.org/10.1177/016327879601900205ArticlePubMed
- 36. Choi HS, Choi JH, Park KH, Joo KJ, Ga H, Ko HJ, et al. Standardization of the Korean version of Patient Health Questionnaire-9 as a Screening instrument for major depressive disorder. J Korean Acad Fam Med. 2007;28(2):114-119.
- 37. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. https://doi.org/10.1046/j.1525-1497.2001.016009606.xArticlePubMedPMC
- 38. Park SJ, Choi HR, Choi JH, Kim K, Hong JP. Reliability and validity of the Korean version of the Patient Health Questionnaire-9 (PHQ-9). Anxiety Mood. 2010;6(2):119-124.PDF
- 39. Chang S, Song M. The validity and reliability of a Korean version of the Summary of Diabetes Self-Care Activities Questionnaire for older patients with type 2 diabetes. J Korean Acad Adult Nurs. 2009;21(2):235-244.
- 40. Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943-950. https://doi.org/10.2337/diacare.23.7.943ArticlePubMed
- 41. Lee SJ, Song M, Im EO. Effect of a health literacy-considered diabetes self-management program for older adults in South Korea. Res Gerontol Nurs. 2017;10(5):215-225. https://doi.org/10.3928/19404921-20170831-03ArticlePubMed
- 42. Ko H, Song M. Senior center based diabetes self-management program: an action research approach. J Korean Gerontol Soc. 2018;38(1):169-185. https://www.kci.go.kr/kciportal/ci/sereArticleSearch/ciSereArtiView.kci?sereArticleSearchBean.artiId=ART002319742
- 43. Jeon E, Park HA. Experiences of patients with a diabetes self-care app developed based on the information-motivation-behavioral skills model: before-and-after study. JMIR Diabetes. 2019;4(2):e11590. https://doi.org/10.2196/11590ArticlePubMedPMC
- 44. Park YJ. The mentors, the social support and patients with diabetes mellitus. J Korean Diabetes. 2019;20(2):112-116. https://doi.org/10.4093/jkd.2019.20.2.112Article
- 45. Qin W, Blanchette JE, Yoon M. Self-efficacy and diabetes self-management in middle-aged and older adults in the United States: a systematic review. Diabetes Spectr. 2020;33(4):315-323. https://doi.org/10.2337/ds19-0051ArticlePubMedPMC
- 46. Jung JG, Chung EY, Kim YJ, Park HJ, Kim AR, Ban YH, et al. Improvement of knowledge, self-efficacy and self-care behaviors among diabetic patients participated in the education program of Sejong center for hypertension and diabetes management. J Agric Med Community Health. 2017;42(4):234-243. https://doi.org/10.5393/JAMCH.2017.42.4.234Article
References
Figure & Data
REFERENCES
Citations

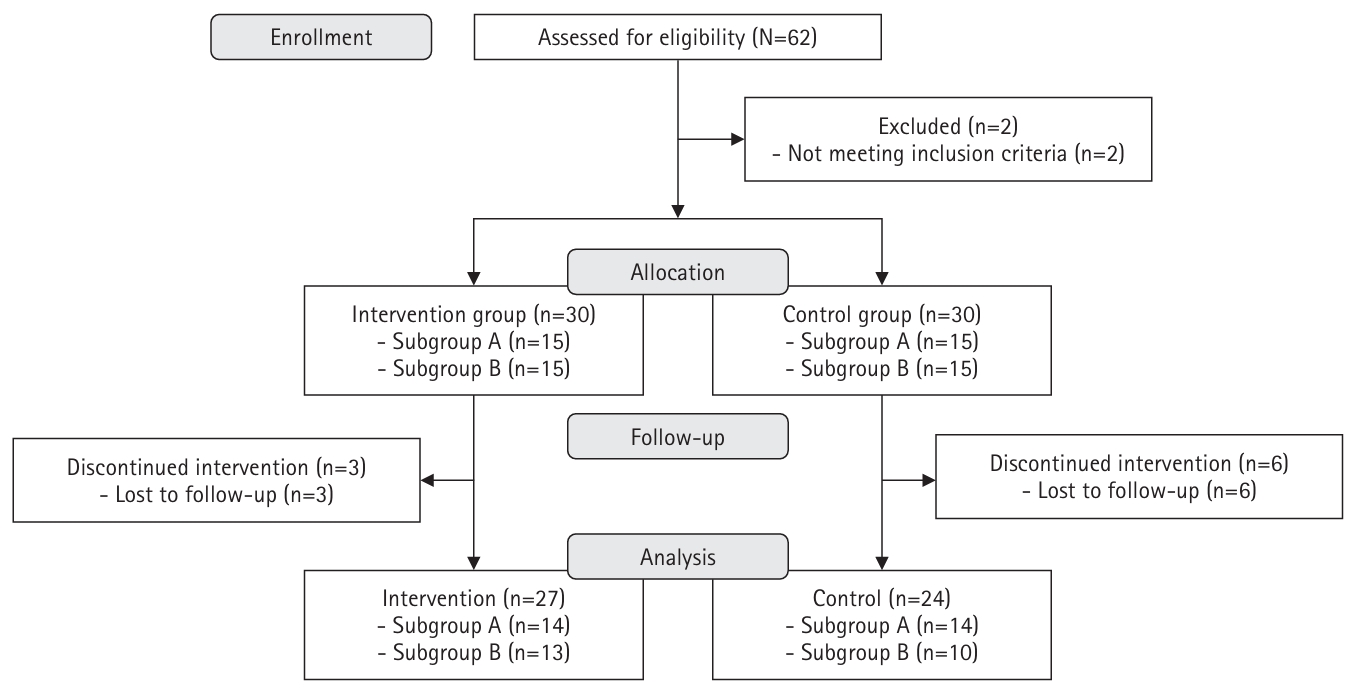
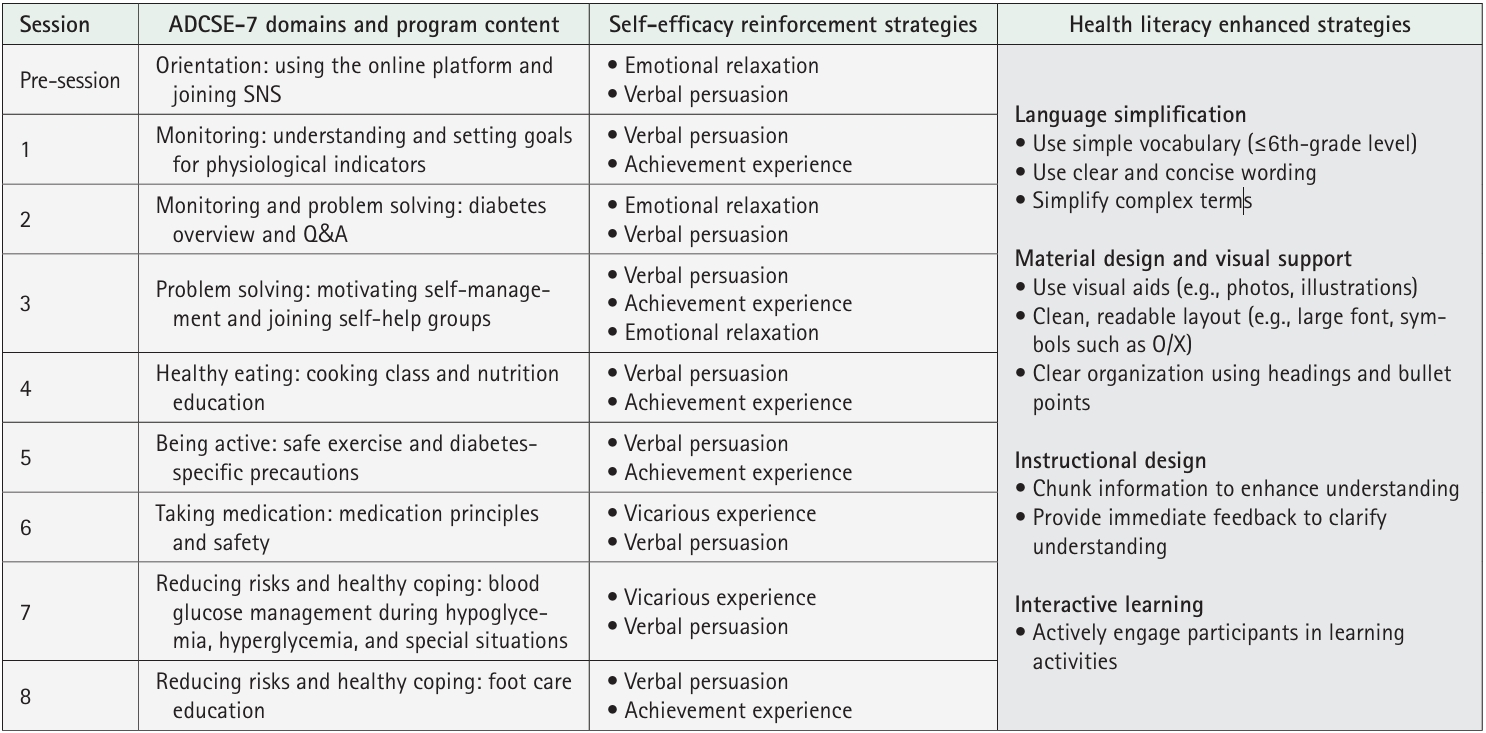
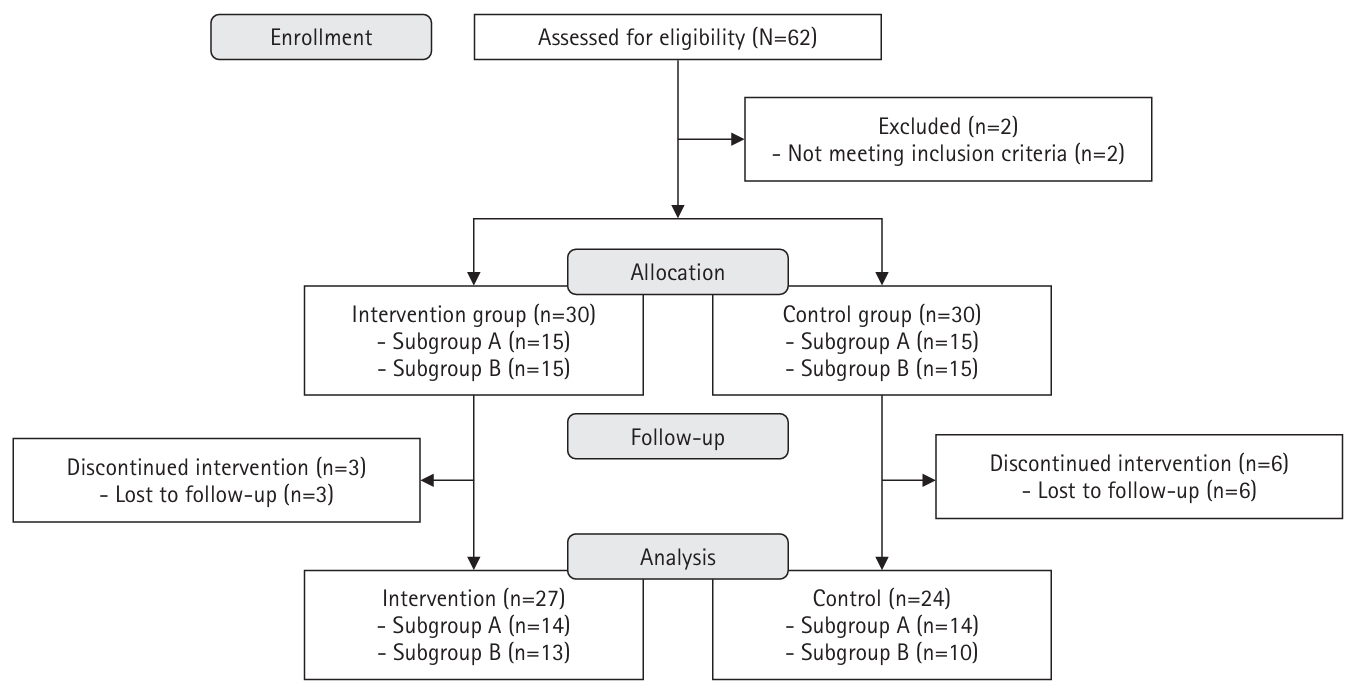
Fig. 1.
Fig. 2.
| Characteristic | Intervention group (n=27) | Control group (n=24) | t or χ2 or U | p |
|---|---|---|---|---|
| Age (yr) | 0.27 | .869 | ||
| <60 | 13 (48.1) | 11 (45.8) | ||
| ≥60 | 14 (51.9) | 13 (54.2) | ||
| Sex | 0.32 | .570 | ||
| Men | 6 (22.2) | 7 (29.2) | ||
| Women | 21 (77.8) | 17 (70.8) | ||
| Education | 1.79 | .408 | ||
| ≤Middle school | 9 (33.3) | 8 (33.3) | ||
| High school | 14 (51.9) | 9 (37.5) | ||
| College or more | 4 (14.8) | 7 (29.2) | ||
| Duration of diabetes (yr) | 0.77 | .802 |
||
| 1–5 | 15 (55.6) | 14 (58.3) | ||
| 6–10 | 7 (25.9) | 4 (16.7) | ||
| ≥11 | 5 (18.5) | 6 (25.0) | ||
| Comorbidities | 0.02 | >.999 | ||
| Yes | 25 (92.6) | 22 (91.7) | ||
| No | 2 (7.4) | 2 (8.3) | ||
| Medication usage | 0.32 | .736 |
||
| Yes | 22 (81.5) | 18 (75.0) | ||
| No | 5 (18.5) | 6 (25.0) | ||
| Experience in diabetes education | 1.85 | .255 |
||
| Yes | 6 (22.2) | 2(8.3) | ||
| No | 21 (77.8) | 22 (91.7) | ||
| Smoking | 0.11 | >.999 | ||
| Yes | 3 (11.1) | 2 (8.3) | ||
| No | 24 (88.9) | 22 (91.7) | ||
| Drinking | 0.91 | .451 |
||
| Yes | 3 (11.1) | 5 (20.8) | ||
| No | 24 (88.9) | 19 (79.2) | ||
| FBG (mg/dL) | 129.63±29.12 | 128.29±25.78 | 321.50 | .966 |
| A1C level (%) | 6.78±0.96 | 6.74±0.84 | 317.00 | .899 |
| SBP (mm Hg) | 126.48±13.01 | 123.54±12.02 | 0.83 | .408 |
| DBP (mm Hg) | 76.15±9.08 | 76.96±8.65 | –0.33 | .746 |
| Total cholesterol (mg/dL) | 158.81±40.99 | 170.46±41.95 | 252.00 | .177 |
| HDL cholesterol (mg/dL) | 42.93±14.95 | 47.71±15.11 | –1.16 | .253 |
| Triglycerides (mg/dL) | 157.07±73.78 | 159.29±80.45 | 322.00 | .974 |
| LDL cholesterol (mg/dL) | 84.70±30.48 | 91.50±34.25 | –0.75 | .457 |
| BMI (kg/m2) | 25.89±5.96 | 24.52±2.71 | 311.50 | .819 |
| Diabetes self-efficacy | 44.85±5.91 | 42.54±6.45 | 223.00 | .056 |
| Diabetes social support | 16.22±7.52 | 15.00±6.99 | 293.00 | .563 |
| Depression | 13.44±4.71 | 16.63±6.47 | 235.50 | .094 |
| DSMB | 72.14±16.89 | 69.79±16.78 | 0.50 | .620 |
| Diet | 19.11±8.28 | 20.58±6.34 | –0.71 | .483 |
| Exercise | 7.78±3.00 | 8.33±3.60 | 294.50 | .578 |
| Blood glucose monitoring | 6.63±5.41 | 4.13±3.68 | 225.00 | .059 |
| Medications | 15.48±5.66 | 13.54±6.09 | 256.00 | .183 |
| Foot care | 23.19±6.98 | 23.21±7.86 | –0.01 | .991 |
| Variable | Intervention group (n=27) | Control group (n=24) | t or U | p |
|---|---|---|---|---|
| FBG (mg/dL) | 113.85±12.91 | 126.75±25.67 | –2.22 | .033 |
| HbA1c level (%) | 6.42±0.75 | 6.39±0.84 | 0.19 | .848 |
| SBP (mm Hg) | 119.33±9.75 | 119.79±11.13 | –0.16 | .877 |
| DBP (mm Hg) | 74.04±8.06 | 73.79±7.51 | 0.11 | .911 |
| Total cholesterol (mg/dL) | 165.00±36.78 | 169.21±47.12 | 322.50 | .491 |
| HDL cholesterol (mg/dL) | 49.48±17.59 | 49.63±14.40 | –0.03 | .975 |
| Triglycerides (mg/dL) | 142.37±80.80 | 143.92±78.06 | 322.50 | .491 |
| LDL cholesterol (mg/dL) | 86.11±30.01 | 93.29±43.89 | 310.50 | .804 |
| BMI (kg/m2) | 25.48±5.98 | 23.73±2.77 | 1.37 | .179 |
| Diabetes self-efficacy | 57.40±5.15 | 51.04±5.69 | 4.19 | <.001 |
| Diabetes social support | 26.19±2.43 | 21.75±4.67 | 4.32 | <.001 |
| Depression | 11.04±1.99 | 14.17±5.19 | 223.50 | .055 |
| DSMB | 89.74±10.57 | 77.96±11.58 | 3.80 | <.001 |
| Diet | 25.67±5.73 | 23.04±5.03 | 1.73 | .090 |
| Exercise | 11.11±2.79 | 9.29±3.48 | 2.07 | .044 |
| Blood glucose monitoring | 11.52±3.49 | 6.13±3.69 | 5.38 | <.001 |
| Medications | 16.11±4.37 | 13.92±5.76 | 267.50 | .184 |
| Foot care | 25.33±6.54 | 25.58±7.25 | –0.13 | .897 |
| Variable | Group | Pre-test | Post-test | t or Z | p | Change (post-pre) | t or U | p |
|---|---|---|---|---|---|---|---|---|
| FBG (mg/dL) | I | 129.63±29.12 | 113.85±12.91 | –3.28 | <.001 |
–15.78±25.69 | 192.00 | .012 |
| C | 128.29±25.78 | 126.75±25.67 | 0.79 | .439 | –1.54±9.60 | |||
| HbA1c level (%) | I | 6.78±0.96 | 6.42±0.75 | –2.85 | .002 |
–0.36±0.61 | 299.00 | .649 |
| C | 6.74±0.84 | 6.39±0.84 | 4.09 | <.001 | –0.37±0.44 | |||
| SBP (mm Hg) | I | 126.48±13.01 | 119.33±9.75 | 2.44 | .022 | –7.15±15.25 | –0.82 | .419 |
| C | 123.54±12.02 | 119.79±11.13 | 1.28 | .214 | –3.75±14.38 | |||
| DBP (mm Hg) | I | 76.15±9.08 | 74.04±8.06 | 1.08 | .289 | –2.11±10.12 | 0.37 | .715 |
| C | 76.96±8.65 | 73.79±7.51 | 1.49 | .149 | –3.17±10.40 | |||
| Total cholesterol (mg/dL) | I | 158.81±40.99 | 165.00±36.78 | –1.57 | .118 |
6.19±36.00 | 256.00 | .203 |
| C | 170.46±41.95 | 169.21±47.12 | 0.17 | .871 | –1.25±37.19 | |||
| HDL cholesterol (mg/dL) | I | 42.93±14.95 | 49.48±17.59 | –2.69 | .012 | 6.56±12.67 | 1.27 | .211 |
| C | 47.71±15.11 | 49.63±14.40 | –0.70 | .492 | 1.92±13.44 | |||
| Triglycerides (mg/dL) | I | 157.07±73.78 | 142.37±80.80 | 0.97 | .342 | –14.7±78.86 | 0.03 | .974 |
| C | 159.29±80.45 | 143.92±78.06 | 1.15 | .262 | –15.38±65.55 | |||
| LDL cholesterol (mg/dL) | I | 84.70±30.48 | 86.11±30.01 | –0.27 | .790 | 1.41±27.13 | –0.05 | .960 |
| C | 91.50±34.25 | 93.29±43.89 | –0.32 | .753 | 1.79±27.59 | |||
| BMI (kg/m2) | I | 25.89±5.96 | 25.48±5.98 | –1.01 | .325 |
–0.41±6.07 | 292.00 | .552 |
| C | 24.52±2.71 | 23.73±2.77 | –3.62 | <.001 |
–0.79±1.31 | |||
| Diabetes self-efficacy | I | 44.85±5.91 | 57.40±5.15 | –12.04 | <.001 | 12.56±5.42 | 184.50 | .008 |
| C | 42.54±6.45 | 51.04±5.69 | –3.89 | <.001 |
8.50±7.25 | |||
| Diabetes social support | I | 16.22±7.52 | 26.19±2.43 | –7.15 | <.001 | 9.96±7.25 | 1.74 | .089 |
| C | 15.00±6.99 | 21.75±4.67 | –5.73 | <.001 | 6.75±5.77 | |||
| Depression | I | 13.44±4.71 | 11.04±1.99 | –3.07 | .002 |
–2.41±3.75 | 311.00 | .810 |
| C | 16.63±6.47 | 14.17±5.19 | –3.02 | .001 |
–2.46±3.34 | |||
| DSMB | I | 72.14±16.89 | 89.74±10.57 | –5.34 | <.001 | 17.59±17.13 | 2.14 | .037 |
| C | 69.79±16.78 | 77.96±11.58 | –2.89 | .008 | 8.17±13.86 | |||
| Diet | I | 19.11±8.28 | 25.67±5.73 | –5.40 | <.001 | 6.56±6.31 | 2.42 | .019 |
| C | 20.58±6.34 | 23.04±5.03 | –2.10 | .047 | 2.46±5.73 | |||
| Exercise | I | 7.78±3.00 | 11.11±2.79 | –6.09 | <.001 | 3.33±2.84 | 2.79 | .007 |
| C | 8.33±3.60 | 9.29±3.48 | –1.45 | .160 | 0.96±3.24 | |||
| Blood glucose monitoring | I | 6.63±5.41 | 11.52±3.49 | –4.41 | <.001 | 4.89±5.77 | 207.50 | .026 |
| C | 4.13±3.68 | 6.13±3.69 | –2.41 | .014 |
2.00±3.68 | |||
| Medications | I | 15.48±5.66 | 16.11±4.37 | –0.75 | .452 |
0.63±4.23 | 287.00 | .461 |
| C | 13.54±6.09 | 13.92±5.76 | –0.88 | .489 |
0.38±3.03 | |||
| Foot care | I | 23.19±6.98 | 25.33±6.54 | –1.12 | .263 |
2.15±8.20 | 321.00 | .959 |
| C | 23.21±7.86 | 25.58±7.25 | –1.46 | .158 | 2.38±7.97 |
Values are presented as number (%) or mean±standard deviation. HbA1c, glycated hemoglobin; BMI, body mass index; DBP, diastolic blood pressure; DSMB, diabetes self-management behavior; FBG, fasting blood glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure. By Fisher’s exact test. By Mann-Whitney U test.
Values are presented as mean±standard deviation. HbA1c, glycated hemoglobin; BMI, body mass index; DBP, diastolic blood pressure; DSMB, diabetes self-management behavior; FBG, fasting blood glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure. By Mann-Whitney U test.
Values are presented as mean±standard deviation. HbA1c, glycated hemoglobin; BMI, body mass index; C, control; DBP, diastolic blood pressure; DSMB, diabetes self-management behavior; FBG, fasting blood glucose; HDL, high-density lipoprotein; I, intervention; LDL, low-density lipoprotein; SBP, systolic blood pressure. By Wilcoxon signed rank test. By Mann-Whitney U test.
 KSNS
KSNS
 E-SUBMISSION
E-SUBMISSION
 ePub Link
ePub Link Cite
Cite