Articles
- Page Path
- HOME > J Korean Acad Nurs > Volume 42(5); 2012 > Article
-
Original Article
- A Meta-analysis of Chemotherapy related Cognitive Impairment in Patients with Breast Cancer
- Jin-Hee Park1, Sun Hyoung Bae2
-
Journal of Korean Academy of Nursing 2012;42(5):644-658.
DOI: https://doi.org/10.4040/jkan.2012.42.5.644
Published online: October 12, 2012
1College of Nursing, Ajou University, Suwon, Korea
2College of Nursing, Yonsei University, Seoul, Korea
1College of Nursing, Ajou University, Suwon, Korea
2College of Nursing, Yonsei University, Seoul, Korea
- Address reprint requests to : Bae, Sun Hyoung San 5, Wonchon-dong, Yeongtong-gu, Suwon 443-721, Korea. Tel: +82-31-219-7019 Fax: +82-31-219-7020 E-mail: baega7695@hanmail.net
Copyright © 2012 Korean Society of Nursing Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Purpose
- The purpose of this study was to evaluate the cognitive effects of chemotherapy in patients with breast cancer.
-
Methods
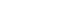
- Using several databases, prospective studies were collected up to August 2011. Of 2,106 publications identified, 12 met the inclusion criteria, and 8 studies were used to estimate the effect size of chemotherapy on cognitive impairment.
-
Results
- Twelve studies were done since 2005 and most of the research was performed in Europe or North America. Eight studies were used to generate effect size across the cognitive domains of attention/concentration, verbal and visual memory, executive function, visuospatial skill, language, and subjective cognitive function. Each of the cognitive domains showed small effect sizes (-0.02 ~ -0.26), indicating diminished cognitive function for the chemotherapy group compared with non-chemotherapy groups.
-
Conclusion
- Finding suggests that breast cancer patients who undergo chemotherapy may experience mild cognitive decline. Further study is needed to generate knowledge and guideline for interventions to address chemotherapy related cognitive impairment in these patients.
* AC=Doxorubicin and cyclophosphamide; ACt=Doxorubicin, cyclophosphamide, and taxane; CAF=Cyclophosphamide, doxorubicin, and 5-fluorouracil; CEA=Cyclophosphamide, epirubicin, and adriamycin; CEF=Cyclophosphamide, epirubicin, and 5-fluorouracil; CMF=Cyclophosphamide, methotrexate, and 5-fluorouracil; EC=Epirubicin and cyclophosphamide; FAC=5-fluorouracil, doxorubicin, and cyclophosphamide; FEA=5-fluorouracil, epirubicin, and adriamycin; FEC=5-fluorouracil, epirubicin, and cyclophosphamide; LH-RH=Luteinizing hormone-releasing hormone; TAC=Taxotere, adriamycin, and cyclophosphamide.
†AVRT=Auditory verbal learning test; COWAT=Controlled oral word association test; CPT=Continuous performance test; CVLT=California verbal learning test; DKEFS=Delis-Kaplan executive function system; PASAT=Paced auditory serial addition test; RAVLT=Rey auditory verbal learning test; RCFT=Rey complex figure test; RVLT=Rey visual learning test; WAIS=Wechsler adult intelligence scale; WMS-III=Wechsler memory scale-version 3; WMS-R=Wechsler memory scale-revised.
‡BDI-II=Beck depression inventory-version 2; CES-D=Center for epidemiological study–depression; CFQ=Cognitive failures questionnaire; EORTC QLQ-C30=European organization for the research and treatment of cancer quality of life questionnaire; FACT-B=Functional assessment of cancer therapy scale - for breast cancer; FACT-ES=Functional assessment of cancer therapy scale – endocrine symptom; FACT-F=Functional assessment of cancer therapy scale – fatigue; FACT-G=Functional assessment of cancer therapy scale - general; FSI=Fatigue symptom inventory; GHQ12=General health questionnaire 12; GPS=General perceived self-efficacy; HADS=Hospital anxiety and depression scale; HSCS=High sensitivity cognitive screen; MASRQ=Multiple ability self-report questionnaire; PAOF=Patient’s assessment of own functioning; POMS=Profile of mood states; PSI=Pittsburgh sleep inventory; PSS=Perceived stress scale; SCF=Subjective cognitive functioning; SLS=Satisfaction with life scale; SSQt=Social support questionnaire of transactions; STAI=State trait anxiety inventory; ZAS=Zung anxiety scale.
§Quality assessment of literature by Scottish Intercollegiate Guidelines Network checklist. ‘+ +’ indicates that all or most of the criteria have been fulfilled. ‘+’ indicates that some of the criteria have been fulfilled. ‘-’ indicates fe or no criteria fulfilled.
Abbreviation: BC=Breast cancer; CCTx=Comparison group who matched with breast cancer treated with chemotherapy; CnonCTx=Comparison group who matched with breast cancer not treated with chemotherapy; CTx=Treated with chemotherapy; DCIS=Ductal carcinoma in situ; HRTx=Treated with hormone therapy; M=mean; nonCTx=Not treated with chemotherapy; RTx=Treated with radiotherapy; SD=Standard deviation; U.K.=United Kingdom; U.S.A.=United States of America.
| Test | No. of studies* | n† | Effect size‡ | Lower 95% CI | Upper 95% CI | Z | p | Nfs |
|---|---|---|---|---|---|---|---|---|
| Attention/concentration | 19 | 1,515 | −0.14 | −0.24 | −0.03 | 2.57 | .010 | 15 |
| Digit span-forward and backward | 3 | 265 | −0.08 | −0.32 | 0.16 | 0.64 | .524 | |
| Digit span-forward | 2 | 127 | −0.24 | −0.73 | 0.25 | 0.96 | .337 | |
| Trails making test-part A | 5 | 392 | −0.06 | −0.27 | 0.14 | 0.61 | .538 | |
| Arithmetic | 4 | 311 | −0.06 | −0.29 | 0.17 | 0.49 | .626 | |
| Letter-number sequencing | 5 | 420 | −0.28 | −0.48 | −0.08 | 2.78 | .005 | |
| Memory | 30 | 2,204 | −0.17 | −0.25 | −0.08 | 3.66 | <.001 | 73 |
| Verbal memory | 20 | 1,602 | −0.17 | −0.28 | −0.07 | 3.19 | .001 | 32 |
| CVLT short delay free recall | 3 | 265 | −0.39 | −0.64 | −0.14 | 3.11 | .002 | |
| CVLT long delay free recall | 3 | 265 | −0.08 | −0.33 | 0.16 | 0.68 | .500 | |
| CVLT long delay recognition | 3 | 265 | -0.06 | -0.30 | 0.19 | 0.47 | .636 | |
| RAVLT immediate recall | 6 | 458 | −0.21 | −0.41 | 0.00 | 1.94 | .052 | |
| RAVLT long delay recall | 5 | 349 | −0.11 | −0.36 | 0.14 | 0.86 | .392 | |
| Visual memory | 10 | 602 | −0.15 | −0.32 | 0.01 | 1.85 | .063 | 0 |
| RCFT immediate recall | 5 | 301 | −0.19 | −0.42 | 0.04 | 1.59 | .113 | |
| RCFT long delay recall | 5 | 301 | −0.12 | −0.35 | 0.11 | 1.04 | .297 | |
| Executive function | 17 | 1,407 | -0.08 | -0.18 | 0.03 | 1.35 | .176 | 0 |
| Digit symbol–coding subset | 4 | 311 | −0.09 | −0.32 | 0.14 | 0.76 | .444 | |
| Digit symbol-symbol search subset | 4 | 311 | −0.09 | −0.32 | 0.14 | 0.80 | .421 | |
| Trails making test-part B | 5 | 392 | −0.09 | −0.30 | 0.11 | 0.90 | .366 | |
| Digit span-backward | 4 | 393 | −0.03 | −0.24 | 0.19 | 0.24 | .812 | |
| Visuospatial skill | 2 | 127 | −0.11 | −0.47 | 0.26 | 0.58 | .562 | − |
| RCFT copy | 2 | 127 | -0.11 | -0.47 | 0.26 | 0.58 | .562 | |
| Language | 3 | 265 | −0.02 | −0.26 | 0.23 | 0.13 | .902 | 0 |
| Boston naming test | 3 | 265 | −0.02 | −0.26 | 0.23 | 0.13 | .902 | |
| Subjective cognitive function | 6 | 474 | −0.26 | −0.44 | −0.07 | 2.76 | .006 | 6 |
- Ahles T. A., Saykin A.J. 2002;Breast cancer chemotherapy-related cognitive dysfunction. Clinical Breast Cancer. 3(Suppl 3):S84–S90. http://dx.doi.org/10.3816/CBC.2002.s.018ArticlePubMed
- Ahles T. A., Saykin A. J., McDonald B. C., Li Y., Furstenberg C. T., Hanscom B. S., et al. 2010;Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. Journal of Clinical Oncology. 28:4434–4440. http://dx.doi.org/10.1200/JCO.2009.27.0827ArticlePubMedPMC
- Bender C. M., Sereika S. M., Berga S. L., Vogel V. G., Brufsky A. M., Paraska K. K., et al. 2006;Cognitive impairment associated with adjuvant therapy in breast cancer. Psycho-Oncology. 15:422–430. http://dx.doi.org/10.1002/pon.964ArticlePubMed
- Braver T. S., Barch D.M. 2002;A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience and Biobehavioral Reviews. 26:809–817. http://dx.doi.org/10.1016/S0149-7634(02)00067-2ArticlePubMed
- Collins B., Mackenzie J., Stewart A., Bielajew C., Verma, S. 2009;Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psycho-Oncology. 18:134–143. http://dx.doi.org/10.1002/pon.1379ArticlePubMed
- Cusimano, A. 2001.Learning disabilities: There is a cure. Pennsylvania, PA: Achieve Publications.
- Debess J., Riis J. Ø., Engebjerg M. C., Ewertz, M. 2010;Cognitive function after adjuvant treatment for early breast cancer: A population-based longitudinal study. Breast Cancer Research and Treatment. 121:91–100. http://dx.doi.org/10.1007/s10549-010-0756-8ArticlePubMedPDF
- DerSimonian R., Laird, N. 1986;Meta-analysis in clinical trials. Controlled Clinical Trials. 7:177–188. http://dx.doi.org/10.1016/0197-2456(86)90046-2ArticlePubMed
- Egger M., Davey Smith G. D., Schneider M., Minder, C. 1997;Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 315:629–634. http://dx.doi.org/10.1136/bmj.315.7109.629ArticlePubMedPMC
- Falleti M. G., Sanfilippo A., Maruff P., Weih L., Phillips K.A. 2005;The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: A meta-analysis of the current literature. Brain and Cognition. 59:60–70. http://dx.doi.org/10.1016/j.bandc.2005.05.001ArticlePubMed
- Fan H. G., Houédé-Tchen N., Yi Q. L., Chemerynsky I., Downie F. P., Sa-bate K., et al. 2005;Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1-and 2-year follow-up of a prospective controlled study. Journal of Clinical Oncology. 23:8025–8032. http://dx.doi.org/10.1200/JCO.2005.01.6550ArticlePubMed
- Gazzaniga M. S., Ivry R. B., Mangun G.R. 2002.Cognitive neuroscience: The biology of the mind. 2nd ed.New York, NY: W.W. Norton & Company.
- Glogoski C., Milligan N. V., Wheatley C.J. 2006.Evaluation and treatment of cognitive dysfunction. In: Pendleton H.M., Schultz- Krohn W., editors. Pedretti’s occupational therapy: Practice skills for physical dysfunction. 6th ed. p. 589–608. St. Louis, MO: Mosby Elsevier.
- Isaki E., Spaulding T. J., Plante, E. 2008;Contributions of language and memory demands to verbal memory performance in language-learning disabilities. Journal of Communication Disorders. 41:512–530. http://dx.doi.org/10.1016/j.jcomdis.2008.03.006ArticlePubMed
- Jansen C. E., Miaskowski C. A., Dodd M. J., Dowling G.A. 2007;A meta-analysis of the sensitivity of various neuropsychological tests used to detect chemotherapy-induced cognitive impairment in patients with breast cancer. Oncology Nursing Forum. 34:997–1005. http://dx.doi.org/10.1188/07.ONF.997-1005ArticlePubMed
- Kim G. D., Chung B. Y., Kim K. H., Byun H. S., Choi E.H. 2011;Analysis of a trend of instrument usage to assess cognitive function of breast cancer patients undergoing chemotherapy. Journal of Korean Oncology Nursing. 11:179–185. http://dx.doi.org/10.5388/jkon.2011.11.3.179ArticlePDF
- Knobf M.T. 2011;Clinical update: Psychosocial responses in breast cancer survivors. Seminars in Oncology Nursing. 27:e1–e14. http://dx.doi.org/10.1016/j.soncn.2011.05.001Article
- Korean National Statistical Office. 2009.Annual report on cause of death statistics. Seoul: Author.
- Lezak M. D., Howieson D. B., Loring D.W. 2004.Neuropsychological assessment. 4th ed.New York, NY: Oxford University Press.
- Matsuda T., Takayama T., Tashiro M., Nakamura Y., Ohashi Y., Shimo-zuma, K. 2005;Mild cognitive impairment after adjuvant chemotherapy in breast cancer patients-evaluation of appropriate research design and methodology to measure symptoms. Breast Cancer. 12:279–287. http://dx.doi.org/10.2325/jbcs.12.279ArticlePubMed
- Myers J.S. 2009;Chemotherapy-related cognitive impairment. Clinical Journal of Oncology Nursing. 13:413–421. http://dx.doi.org/10.1188/09.CJON.413-421ArticlePubMed
- National Evidence-based Healthcare Collaborating Agency. 2011.NE-CA’s guidance for systematic review for new health technology assessment. Seoul: Author.
- Nelson C. J., Nandy N., Roth A.J. 2007;Chemotherapy and cognitive deficits: Mechanisms, findings, and potential interventions. Palliative and Supportive Care. 5:273–280. http://dx.doi.org/10.1017/S1478951507000442ArticlePubMed
- Noal S., Levy C., Hardouin A., Rieux C., Heutte N., Ségura C., et al. 2011;One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. International Journal of Radiation Oncology, Biology, Physics. 81:795–803. http://dx.doi.org/10.1016/j.ijrobp.2010.06.037ArticlePubMed
- Ouimet L. A., Stewart A., Collins B., Schindler D., Bielajew, C. 2009;Measuring neuropsychological change following breast cancer treatment: An analysis of statistical models. Journal of Clinical and Experimental Neuropsychology. 31:73–89. http://dx.doi.org/10.1080/13803390801992725ArticlePubMed
- Quesnel C., Savard J., Ivers, H. 2009;Cognitive impairments associated with breast cancer treatments: Results from a longitudinal study. Breast Cancer Research and Treatment. 116:113–123. http://dx.doi.org/10.1007/s10549-008-0114-2ArticlePubMedPDF
- Rosenthal, R. 1991.Meta-analytic procedures for social research. Newbury Park, CA: Sage Publications.
- Scottish Intercollegiate Guidelines Network. 2011;SIGN 50: A guideline developer's handbook. Retrieved January, 11, 2012, from. http://www.sign.ac.uk/pdf/sign50.pdf
- Stewart A., Bielajew C., Collins B., Parkinson M., Tomiak, E. 2006;A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. The Clinical Neuropsychologist. 20:76–89. http://dx.doi.org/10.1080/138540491005875ArticlePubMed
- Tager F. A., McKinley P. S., Schnabel F. R., El-Tamer M., Cheung Y. K., Fang Y., et al. 2010;The cognitive effects of chemotherapy in post- menopausal breast cancer patients: A controlled longitudinal study. Breast cancer Research and Treatment. 123:25–34. http://dx.doi.org/10.1007/s10549-009-0606-8ArticlePDF
REFERENCES
Figure & Data
REFERENCES
Citations

- The Experience of Chemotherapy Related Cognitive Impairment in Patients with Cancer
Pok Ja Oh, Ji Hyun Kim
Asian Oncology Nursing.2022; 22(1): 1. CrossRef - Effects of smart-care services program for breast cancer survivors
Bok Yae Chung, Sung Jung Hong
The Journal of Korean Academic Society of Nursing Education.2021; 27(2): 95. CrossRef - Changes of Cognitive Function and Fatigue following Chemotherapy in Patients with Gastrointestinal Cancer: A Prospective Controlled Study
Pok-Ja Oh, Sun Mi Moon
Asian Oncology Nursing.2019; 19(3): 126. CrossRef - Effects of compensatory cognitive training intervention for breast cancer patients undergoing chemotherapy: a pilot study
Jin-Hee Park, Yong Sik Jung, Ku Sang Kim, Sun Hyoung Bae
Supportive Care in Cancer.2017; 25(6): 1887. CrossRef - Impact of Cognitive Function and Cancer Coping on Quality of Life among Women with Post-chemotherapy Breast Cancer
Yoon Jung Kim, Sook Jung Kang
Korean Journal of Women Health Nursing.2016; 22(3): 182. CrossRef - Chemotherapy-related Cognitive Impairment and Quality of Life in People with Colon Cancer: The Mediating Effect of Psychological Distress
Pok Ja Oh, Jeong Hye Kim
Journal of Korean Academy of Nursing.2016; 46(1): 19. CrossRef - QLU-C10D: a health state classification system for a multi-attribute utility measure based on the EORTC QLQ-C30
M. T. King, D. S. J. Costa, N. K. Aaronson, J. E. Brazier, D. F. Cella, P. M. Fayers, P. Grimison, M. Janda, G. Kemmler, R. Norman, A. S. Pickard, D. Rowen, G. Velikova, T. A. Young, R. Viney
Quality of Life Research.2016; 25(3): 625. CrossRef - The Impact of Cancer on Psychological and Social Outcomes
Daniel Sj Costa, Rebecca Mercieca‐bebber, Claudia Rutherford, Liam Gabb, Madeleine T King
Australian Psychologist.2016; 51(2): 89. CrossRef - Prevalence and Characteristics of Chemotherapy-related Cognitive Impairment in Patients with Breast Cancer
Jin-Hee Park, Sun Hyoung Bae, Yong-Sik Jung, Young-Mi Jung
Journal of Korean Academy of Nursing.2015; 45(1): 118. CrossRef
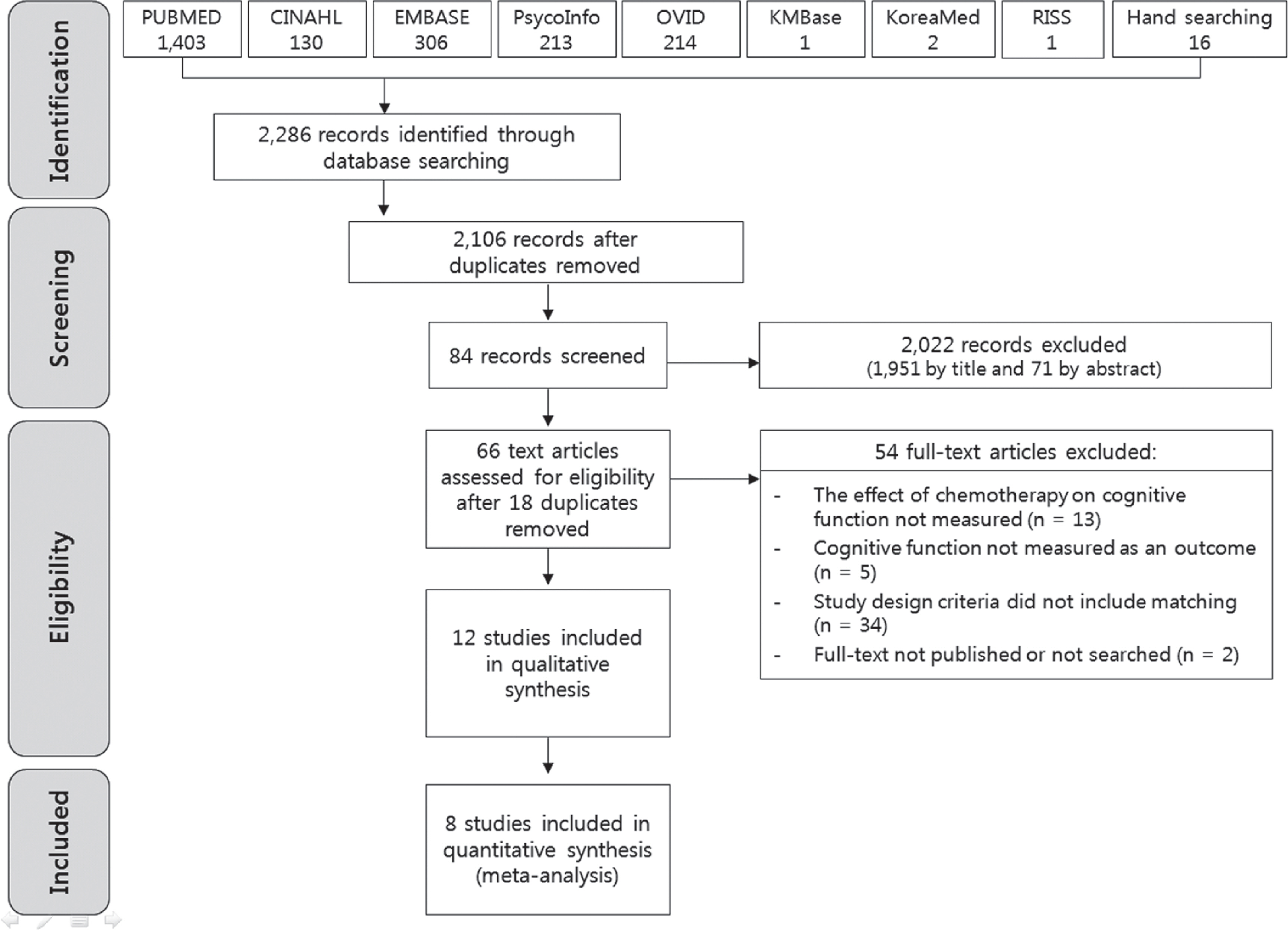
Figure 1.
| Study ID C | Country | Sample | Age (year), M±SD | Education level | Staging and treatment* | Measurement | Type of neuropsychological test† | Other variables and tools‡ | Quality assessment§ | Included in meta-analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahles et al. (2010) | U.S.A. | BC, CTx (n=60) | 51.7±7.1 | No differences among groups | Staging: Stage 0, I, II, III (Exact percentages were not reported) Standard-dose chemotherapy: 30% AC, 36.7% AC plus paciltaxel, 3.3% AC plus taxotere, 1.8% CAF, 11.7% CMF, 16.7% FEC Local treatment: 81% RTx HRTx: 80% tamoxifen or anastrozole | T1: Baseline (before CTx) T2: 1 month T3: 6 months T4: 18 months after CTx | CVLT; Color-word interference test (DKEFS); Digit symbol-coding; Distractibility, reaction time (CPT); Faces I & II; Grooved pegboard; Logical memory I & II; PASAT; Sorting test; Trail making test; Verbal fluency test; Vocabulary (WAIS) | Anxiety (STAI) Depression (CES-D) Fatigue (FSI) Subjective cognitive ability (MASRQ) | + | Subjective cognitive function |
| BC, nonCTx (n=72) | 56.6±8.3 | Staging: Stage 0, I, II, III (Exact percentages were not reported) Local treatment: 72% RTx HRTx: 66% tamoxifen or anastrozole | ||||||||
| Comparison (n=45) | 52.9±10.0 | Healthy women | ||||||||
| Bender et al. (2006) Collins et al. | U.S.A Canada | BC, CTx (n=19) | 40.1±6.5 | No differences among groups No differences | Staging: 32% Stage I, 68% Stage II Standard-dose chemotherapy: 40% AC, 40% AC plus a taxane, 20% CMF | T1: Baseline T2: 1 week T3: 12 months after CTx | Digit vigilance test; Four word short memory test; RAVLT; RCFT; Trail making test-B | Anxiet (POMS) Depression (BDI-II) Fatigue (POMS) Subjective cognitive ability (PAOF) | − | Objective & subjective cognitive function Objective |
| BC, nonCTx (n=15) | 44.1±3.5 | Staging: 40% Stage I, 60% Stage II Standard-dose chemotherapy: | ||||||||
| Standard-dose chemotherapy: 33% AC, 42% AC plus a taxane, 25% doxorubicin and a taxane HRTx: 100% tamoxifen | ||||||||||
| BC (n=12) | 44.5±4.2 | Staging: 100% DCIS | ||||||||
| Collins et al. (2009) | Canada | BC, CTx (n=53) | 57.9±3.7 | No differences between groups | Staging: 28.3% Stage I, 67.9% Stage II, 3.8% Stage III Standard-dose chemotherapy: 24.5% AC, 5.7% AC plus taxol, 9.4% CEF, 3.7% EC plus taxol, 5.7% FAC, 49.1% FEC, 1.9% Adriamycin and cisplatin HRTx: 11% tamoxifen, 4% arimidex, 2% letrozole | T1: Baseline(before CTx) T2: 1 month T3: 12 months after CTx | Arithmetic; Block design; Boston naming test; CVLT; Consonant trigrams; Phonemic word fluency(COWAT); Digit span-forward + backward; Digit symbol, Coding; Family pictures II; Grooved pegboard; Letter-number sequencing; PASAT; RVLT; Spatial span; Trail making test A or B; Wisconsin card sorting test | Anxiety (POMS) Depression (POMS) Fatigue (POMS) | + + | Objective cognitive function |
| BC, nonCTx (n=40) | 57.6±4.0 | Staging: 87.5% Stage I, 12.5% Stage II | ||||||||
| HRTx: 63% tamoxifen, 27% arimidex, 10% switched (tamoxifen to arimidex) | ||||||||||
| Debess et al. (2010) | Denmark | BC, CTx (n=75) BC, nonCTx (n=26) BC(n=19) | 47.2 (Range: 29~59) 56.2 (Range: 44~59) 49.7 (Range: 39~59) | No differences among groups | Staging was not reported Standard-dose chemotherapy: 100% CEF Local treatment: 69% RTx Staging was not reported Local treatment: 65% RTx HRTx: tamoxifen 100% Staging was not reported Local treatment: 53% RTx | T1: Baseline (before CTx) T2: 1 month after CTx | Visual verbal learning test; Concept shifting test; Stroop color word interference test; Letter–digit coding test | Anger (POMS) Confusion (POMS) Coping capacity (GPS) Depression (POMS) Fatigue (POMS) Quality of life (EORTC QLQ-C30) Subjective cognitive ability (SCF) Tension (POMS) | + + | No |
| Comparison (n=208) | 48.1 (Range: 28~59) | Healthy women | ||||||||
| Fan et al. (2005) | Canada | BC, CTx (n=104) | Median=48 | No differences between groups | Staging was not reported Standard-dose chemotherapy: 19% AC, 63% CEF, 18% other Local treatment: RTx, 6.7% at T1, 68.1% at T2, 65.4% at T3 HRTx: Tamoxifen or anastrozole, 4.8% at T1, 62.6% at T2, 66.7% at T3 | T1: Baseline (before CTx) T2: 1 year T3: 2 years after CTx | Conner’s continuous performance test; Trail making test | Fatigue (FACT-F) Menopausal symptoms (FACT-ES) Quality of life (FACT-G) Subjective cognitive ability (HSCS) | + | No |
| Comparison (n=102) | Median=47 | Healthy women | ||||||||
| Mehlsen et al. (2009) | Denmark | BC, CTx (n=36) Comparison (n=14) | 48.6±8.0 39.3±11.7 | Significant differences between groups (p=.001) | Staging was not reported Standard-dose chemotherapy: 100% CEF Healthy people (6 males, 11 females) | T1: Baseline (before CTx) T2: 4-6 weeks after CTx (Comparison: 3-4 months) | Arithmetic; Coding & symbol search; Digit span, Forward or Backward; Letter-number sequencing; Logical memory; RAVLT; RCFT; Stroop test; Trail making test A & B; Word fluency test | Confusion (POMS) Depression (BDI-II) Life satisfaction (SLS) Sleep quality (PSI) Social support (SSQT) Stress (PSS) | + + | Objective cognitive function |
| Noal et al. (2011) | France France | BC, CTx (n=161) | Median=53 (Range: 31-71) | No differences between groups | Staging was not reported Standard-dose chemotherapy: 54% FEC, 30% FEC plus docetaxel, 16% other Local treatment: 100% RTx HRTx: 81% tamoxifen, anastrozole, or tamo+LH-RH analogue | T1: Baseline T2: 3 weeks after baseline T3: End of RTx T4: 4 months T5: 12 months after RTx | Digit span-forward or backward; RAVLT; Trail making test | Quality of life(FACT-G) Fatigue(FACT-F) Anxiety(HADS) Depression(HADS) | + + | No |
| BC, nonCTx (n=141) | Median=58 (Range: 36~84) | Staging was not reported Local treatment: 100% RTx HRTx: 89% tamoxifen, anastrozole, or tamo+LH-RH analogue 89% | ||||||||
| Ouimet et al. (2009) | Canada | BC, CTx (n=49) BC, nonCTx (n=46) Comparison (n=28) | 57.5±3.9 57.5±4.2 59.4±4.1 | Significant differences among groups (p=.014) | Staging: Stage I, II (Exact percentages were not reported) Standard-dose chemotherapy: CEF, FAC, FEC (Exact percentages were not reported) Staging: Stage I, II (Exact percentages were not reported) HRTx: not reported Healthy women | T1: Baseline (before CTx) T2: End of CTx (approximately within 6 months) | Arithmetic; Block design; Boston naming test; CVLT; Consonant trigrams; COWAT; Digit span, forward + backward; Digit symbol-coding & symbol coding; Family pictures; Grooved pegboard; Letter–number sequencing; Logical memory; PASAT; RVLT; Spatial span; Trail making test A & B | Psychological distress (POMS) | − | Objective cognitive function |
| Quesnel et al. (2009) | Canada | BC, CTx (n=41) | 50.3±7.2 | No differences among groups | Staging: 34.1% Stage I, 48.8% Stage II, 17.1% Stage III Standard-dose chemotherapy: 56.1% AC, 14.6% FEC, 29.3% TAC Local treatment: 100% RTx HRTx: 75.6% | T1: Baseline (before CTx or RTx) T2: Post CTx or RTx T3: 3 months after CTx or RTx | Digit span; RAVLT; RCFT; Ruff 2 & 7 test; Symbol digit modalities test; Trail making test A & B; Verbal fluency test; Visual memory span (WMS-R) | Quality of life (EORTC QLQ-C30) Subjective cognitive ability (CFQ) | + + | Objective & subjective cognitive function |
| BC, nonCTx (n=40) | 57.7±4.9 | Staging: 90.0% Stage I, 10.0% Stage II HRTx: 77.5% | ||||||||
| Comparison (n=45); CCTx (n=23); CnonCTx (n=22) | CCTx, 47.9±7.4; CnonCTx, 55.0±7.1 | Healthy women | ||||||||
| Shilling et al. (2005) | U.K. | BC, CTx (n=50) Comparison (n=43) | 51.1±8.6 52.3±5.8 | No differences between groups | Staging was not reported Standard-dose chemotherapy: 2% AC, 2% CMF, 84% FEC, 12% FEC plus docetaxel HRTx: 28.0% Healthy women HRTx: 51.2% | T1: Baseline (before CTx) T2: 1 month after CTx | Digit span; Letter cancellation; Letter-number sequencing; Logical memory; RAVLT; RCFT; Spatial span; Stroop test | Fatigue (FACT-F) General health (GHQ12) Menopausal symptoms (FACT-ES) Quality of life (FACT-B) Subjective cognitive ability (CFQ) | ) + | Objective & subjective cognitive function |
| Tager et al. (2010) | U.S.A. | BC, CTx (n=30) BC, nonCTx (n=31) | 60.3±5.6 61.1±6.2 | No differences between groups | Staging: 30% Stage I, 70% Stage II Standard-dose chemotherapy: 23.3% AC, 46.7% ACT, 30.0% CMF Local treatment: 56.7% RTx HRTx: 40% Staging: 35.5% DCIS, 51.6% Stage I, 12.9% Stage II Local treatment: 64.5% RTx HRTx: 64.5% | T1: Baseline (before CTx) T2: 1 month T3: 6 months after CTx | Arithmetic; Benton visual retention test; Boston naming test; Busckle selective reminding test; COWAT; Digit span; Digit symbol; Finger tapping test; Grooved pegboard; Letter–number sequencing; RCFT; Trail making test | Depression (BDI-II) Anxiety (ZAS) Subjective cognitive ability (interview) | y + | No |
| Vearncombe et al.(2009) | Australia | BC, CTx (n=136) BC, nonCTx (n=21) | 49.4±7.9 54.0±8.2 | No differences between groups | Staging: 27.2% Stage I, 72.8% Stage II Standard-dose chemotherapy: 5.1% AC, 19.7% AC plus taxol/ taxotere, 8.9% CAF, 3.2% CEA, 0.6% CMF, 44.6% FEC, 3.2% FEC plus taxotere, 0.6% FEA, 0.6% Cyclophosphamide plus taxotere Staging: 81% Stage I, 19% Stage II Local treatment: not reported HRTx: not reported | T1: Baseline (before CTx) T2: 1 month after CTx | AVLT; COWAT; Digit Span-backward; Card sorting task (DKEFS); Matrix reasoning (WAIS-III); Purdue pegboard; Stroop test; Symbol digit modalities test; Test of everyday attention-visual elevator & telephone search; Visual reproduction (WMS-III) | Anxiety and depression (HADS) Fatigue (FACT-F) Quality of life (FACT-B) | + | Objective cognitive function |
| Characteristics | Categories | n (%) | Range |
|---|---|---|---|
| Publication year | 2005 | 2 (16.7) | |
| 2006 | 1 (8.3) | ||
| 2009 | 5 (41.7) | ||
| 2010 | 3 (25.0) | ||
| 2011 | 1 (8.3) | ||
| Region | North America | 7 (58.4) | |
| Europe | 4 (33.3) | ||
| Other | 1 (8.3) | ||
| Sample size | <50 | 1 (8.3) | 46-328 |
| 50-100 | 4 (33.3) | ||
| ≥101 | 7 (58.4) | ||
| Mean age of study participants (year) | <50 | 3 (30.0) | |
| 50-60 | 6 (60.0) | ||
| ≥61 | 1 (10.0) | ||
| Not reported | 2 | ||
| Instrument of subjective cognitive ability | Cognitive failures questionnaire | 2 (33.3) | |
| Multiple ability self-report questionnaire | 1 (16.7) | ||
| Patient's assessment of own functioning | 1 (16.7) | ||
| Subjective cognitive functioning | 1 (16.7) | ||
| Interview | 1 (16.7) | ||
| Not measured | 6 | ||
| Post-test period after treatment (months) | <1 | 3 (25.0) | 0.25-12 |
| 1-3 4 | 7 2 (58.4) (16.7) | ||
| ≥4 | 2 (16.7) | ||
| Follow up period after treatment (months) | <6 6 12 | 5 4 (41.7) (33.3) | 1-24 |
| 6-12 13 | 4 3 (33.3) (25.0) | ||
| ≥13 | 3 (25.0) |
| Test | No. of studies* | n |
Effect size |
Lower 95% CI | Upper 95% CI | Z | p | Nfs |
|---|---|---|---|---|---|---|---|---|
| Attention/concentration | 19 | 1,515 | −0.14 | −0.24 | −0.03 | 2.57 | .010 | 15 |
| Digit span-forward and backward | 3 | 265 | −0.08 | −0.32 | 0.16 | 0.64 | .524 | |
| Digit span-forward | 2 | 127 | −0.24 | −0.73 | 0.25 | 0.96 | .337 | |
| Trails making test-part A | 5 | 392 | −0.06 | −0.27 | 0.14 | 0.61 | .538 | |
| Arithmetic | 4 | 311 | −0.06 | −0.29 | 0.17 | 0.49 | .626 | |
| Letter-number sequencing | 5 | 420 | −0.28 | −0.48 | −0.08 | 2.78 | .005 | |
| Memory | 30 | 2,204 | −0.17 | −0.25 | −0.08 | 3.66 | <.001 | 73 |
| Verbal memory | 20 | 1,602 | −0.17 | −0.28 | −0.07 | 3.19 | .001 | 32 |
| CVLT short delay free recall | 3 | 265 | −0.39 | −0.64 | −0.14 | 3.11 | .002 | |
| CVLT long delay free recall | 3 | 265 | −0.08 | −0.33 | 0.16 | 0.68 | .500 | |
| CVLT long delay recognition | 3 | 265 | -0.06 | -0.30 | 0.19 | 0.47 | .636 | |
| RAVLT immediate recall | 6 | 458 | −0.21 | −0.41 | 0.00 | 1.94 | .052 | |
| RAVLT long delay recall | 5 | 349 | −0.11 | −0.36 | 0.14 | 0.86 | .392 | |
| Visual memory | 10 | 602 | −0.15 | −0.32 | 0.01 | 1.85 | .063 | 0 |
| RCFT immediate recall | 5 | 301 | −0.19 | −0.42 | 0.04 | 1.59 | .113 | |
| RCFT long delay recall | 5 | 301 | −0.12 | −0.35 | 0.11 | 1.04 | .297 | |
| Executive function | 17 | 1,407 | -0.08 | -0.18 | 0.03 | 1.35 | .176 | 0 |
| Digit symbol–coding subset | 4 | 311 | −0.09 | −0.32 | 0.14 | 0.76 | .444 | |
| Digit symbol-symbol search subset | 4 | 311 | −0.09 | −0.32 | 0.14 | 0.80 | .421 | |
| Trails making test-part B | 5 | 392 | −0.09 | −0.30 | 0.11 | 0.90 | .366 | |
| Digit span-backward | 4 | 393 | −0.03 | −0.24 | 0.19 | 0.24 | .812 | |
| Visuospatial skill | 2 | 127 | −0.11 | −0.47 | 0.26 | 0.58 | .562 | − |
| RCFT copy | 2 | 127 | -0.11 | -0.47 | 0.26 | 0.58 | .562 | |
| Language | 3 | 265 | −0.02 | −0.26 | 0.23 | 0.13 | .902 | 0 |
| Boston naming test | 3 | 265 | −0.02 | −0.26 | 0.23 | 0.13 | .902 | |
| Subjective cognitive function | 6 | 474 | −0.26 | −0.44 | −0.07 | 2.76 | .006 | 6 |
* AC=Doxorubicin and cyclophosphamide; ACt=Doxorubicin, cyclophosphamide, and taxane; CAF=Cyclophosphamide, doxorubicin, and 5-fluorouracil; CEA=Cyclophosphamide, epirubicin, and adriamycin; CEF=Cyclophosphamide, epirubicin, and 5-fluorouracil; CMF=Cyclophosphamide, methotrexate, and 5-fluorouracil; EC=Epirubicin and cyclophosphamide; FAC=5-fluorouracil, doxorubicin, and cyclophosphamide; FEA=5-fluorouracil, epirubicin, and adriamycin; FEC=5-fluorouracil, epirubicin, and cyclophosphamide; LH-RH=Luteinizing hormone-releasing hormone; TAC=Taxotere, adriamycin, and cyclophosphamide. †AVRT=Auditory verbal learning test; COWAT=Controlled oral word association test; CPT=Continuous performance test; CVLT=California verbal learning test; DKEFS=Delis-Kaplan executive function system; PASAT=Paced auditory serial addition test; RAVLT=Rey auditory verbal learning test; RCFT=Rey complex figure test; RVLT=Rey visual learning test; WAIS=Wechsler adult intelligence scale; WMS-III=Wechsler memory scale-version 3; WMS-R=Wechsler memory scale-revised. ‡BDI-II=Beck depression inventory-version 2; CES-D=Center for epidemiological study–depression; CFQ=Cognitive failures questionnaire; EORTC QLQ-C30=European organization for the research and treatment of cancer quality of life questionnaire; FACT-B=Functional assessment of cancer therapy scale - for breast cancer; FACT-ES=Functional assessment of cancer therapy scale – endocrine symptom; FACT-F=Functional assessment of cancer therapy scale – fatigue; FACT-G=Functional assessment of cancer therapy scale - general; FSI=Fatigue symptom inventory; GHQ12=General health questionnaire 12; GPS=General perceived self-efficacy; HADS=Hospital anxiety and depression scale; HSCS=High sensitivity cognitive screen; MASRQ=Multiple ability self-report questionnaire; PAOF=Patient’s assessment of own functioning; POMS=Profile of mood states; PSI=Pittsburgh sleep inventory; PSS=Perceived stress scale; SCF=Subjective cognitive functioning; SLS=Satisfaction with life scale; SSQt=Social support questionnaire of transactions; STAI=State trait anxiety inventory; ZAS=Zung anxiety scale. §Quality assessment of literature by Scottish Intercollegiate Guidelines Network checklist. ‘+ +’ indicates that all or most of the criteria have been fulfilled. ‘+’ indicates that some of the criteria have been fulfilled. ‘-’ indicates fe or no criteria fulfilled. Abbreviation: BC=Breast cancer; CCTx=Comparison group who matched with breast cancer treated with chemotherapy; CnonCTx=Comparison group who matched with breast cancer not treated with chemotherapy; CTx=Treated with chemotherapy; DCIS=Ductal carcinoma in situ; HRTx=Treated with hormone therapy; M=mean; nonCTx=Not treated with chemotherapy; RTx=Treated with radiotherapy; SD=Standard deviation; U.K.=United Kingdom; U.S.A.=United States of America.
CI=Confidence interval; CVLT=California verbal learning test; Nfs=Fail-safe N; RAVLT=Rey auditory verbal learning test; RCFT=Rey complex figure test. *The number of studies included per cognitive domain; Total number of participants per effect size; Weighted standardized mean difference.
 KSNS
KSNS
 E-SUBMISSION
E-SUBMISSION
 Cite
Cite