Articles
- Page Path
- HOME > J Korean Acad Nurs > Volume 41(4); 2011 > Article
-
Original Article
- Monitoring the Use of Health-Related Quality of Life Measurements in Korean Studies of Patients with Diabetes
- Eun-Hyun Lee, Chun-Ja Kim, Soo-Yeon Cho, Hyun-Ju Chae, Sunhee Lee, Eun Jung Kim
-
Journal of Korean Academy of Nursing 2011;41(4):558-567.
DOI: https://doi.org/10.4040/jkan.2011.41.4.558
Published online: August 31, 2011
1Associate Professor, Graduate School of Public Health, Ajou University, Suwon, Korea.
2Assistant Professor, College of Nursing, Ajou University, Suwon, Korea.
3Research Assistant, Graduate School of Public Health, Ajou University, Suwon, Korea.
4Part-time Lecturer, College of Nursing, Sungshin Women's University, Seoul, Korea.
5Research Fellow, Yonsei University, Nursing Policy Research Institute, Seoul, Korea.
6Full-time Lecturer, College of Nursing, Eulji University, Seongnam, Korea.
- Address reprint requests to: Kim, Chun-Ja. College of Nursing, Ajou University, San 5 Woncheon-dong, Yeongtong-gu, Suwon 443-721, Korea. Tel: +82-31-219-7017, Fax: +82-31-219-7020, ckimha@ajou.ac.kr
© 2011 Korean Society of Nursing Science
Abstract
-
Purpose
- The purpose of this study was to monitor the use of health-related quality of life (HRQOL) instruments in Korean studies of patients with diabetes.
-
Methods
- Of 86 Korean studies initially identified, 17 studies met the inclusion criteria. For each study, a description of the instrument and its psychometric properties were monitored by the Instrument Review Criteria of the Scientific Advisory Committee. These criteria include conceptual definition, attributes, taxonomy, reliability, validity, responsiveness, administrative mode, and language adaptations.
-
Results
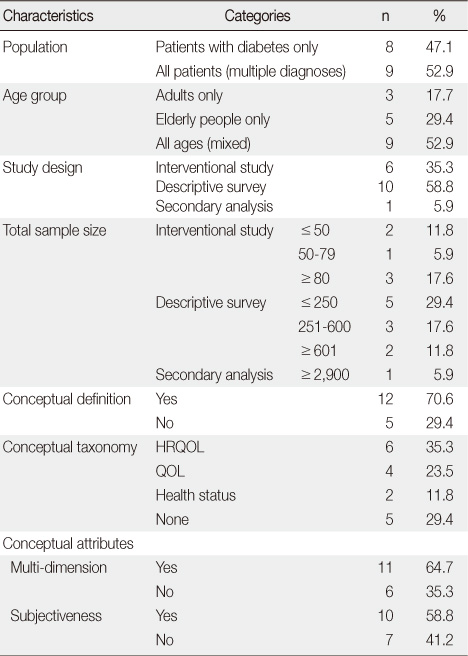
- Five generic and one diabetes specific type questionnaires were identified from the 17 studies. Of those studies, conceptual definitions with the attributes of multi-dimension and subjectiveness were provided for 11 studies (71%). In the analysis of conceptual taxonomy, only 6 studies were classified as HRQOL, while other studies were done as QOL or health status. In monitoring of psychometric properties, reliability, validity, and responsiveness were reported for 88.2%, 64.7%, and 29.4%, respectively. One generic instrument was developed with a Korean population, while the other instruments were developed for Western countries. However, language adaptations were performed for only a few of the instruments.
-
Conclusion
- The psychometric properties including responsiveness of most instruments warrants further research, and the development of diabetes-specific HRQOL measurements should be sought to facilitate intervention outcomes across Korean studies of patients with diabetes.
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0003737).
- 1. Diabetes statistics. American Diabetes Association. 2011;01 26 Retrieved February 18, 2011. from http://www.diabetes.org/diabetes-basics/diabetes-statistics/?utm_source=WWW&utm_medium=Drop-DownDB&utm_content=Statistics&utm_campaign=CON.
- 2. Boyer JG, Earp JA. The development of an instrument for assessing the quality of life of people with diabetes: Diabetes-39. Medical Care. 1997;35:440–453. doi: 10.1097/00005650-199705000-00003.ArticlePubMed
- 3. Brazier J, Jones N, Kind P. Testing the validity of the Euroqol and comparing it with the SF-36 health survey questionnaire. Quality of Life Research. 1993;2:169–180. doi: 10.1007/BF00435221.ArticlePubMedPDF
- 4. Brooks R, Rabin R, de Charro F. The measurement and validation of health status using EQ-5D: A European perspective. 2003;Dordrecht, Kleuer Academic Publishers.
- 5. El Achhab Y, Nejjari C, Chikri M, Lyoussi B. Disease-specific health-related quality of life instruments among adults diabetic: A systematic review. Diabetes Research and Clinical Practice. 2008;80:171–184.ArticlePubMed
- 6. Fayers PM, Machin D. Quality of life: The assessment, analysis and interpretation of patient-related outcomes. 2007;2nd ed. West Sussex, John Wiley & Sons.
- 7. Fryback DG. Advancing social science theory - the importance of common metrics: Measuring health-related quality of life. Workshop conducted at the meeting of The National Academies. 2010;02;Washington, DC, Division of Behavioral and Social Sciences and Education.
- 8. Garratt AM, Ruta DA, Abdalla MI, Russell IT. SF-36 health survey questionnaire: II. Responsiveness to changes in health status in four common clinical conditions. Quality in Health Care. 1994;3:186–192.ArticlePubMedPMC
- 9. Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA. 1994;272:619–626. doi: 10.1001/jama.272.8.619.ArticlePubMed
- 10. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Annals of Internal Medicine. 1993;118:622–629.ArticlePubMed
- 11. Hammond GS, Aoki TT. Measurement of health status in diabetic patients. Diabetes Care. 1992;15:469–477. doi: 10.2337/diacare.15.4.469.PubMed
- 12. Hays RD, Anderson R, Revicki D. Psychometric considerations in evaluating health-related quality of life measures. Quality of Life Research. 1993;2:441–449. doi: 10.1007/BF00422218.ArticlePubMedPDF
- 13. Jacobson A, Barofsky I, Cleary P, Rand L. Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). Diabetes Care. 1988;11:725–732.ArticlePubMedPDF
- 14. Kim MH, Cho YS, Uhm WS, Kim S, Bae SC. Cross-cultural adaptation and validation of the Korean version of the EQ-5D in patients with rheumatic diseases. Quality of Life Research. 2005;14:1401–1406. doi: 10.1007/s11136-004-5681-z.ArticlePubMedPDF
- 15. Korea Centers for Disease Control and Prevention. 2007 National health survey: The 4th Korea national health and nutrition examination survey. 2008;Seoul, Author.
- 16. Lee EH. Development and psychometric evaluation of a quality of life scale for Korean patients with cancer(C-QoL). Journal of Korean Academy of Nursing. 2007;37:324–333.ArticlePubMedPDF
- 17. Lohr KN, Aaronson NK, Alonso J, Burnam MA, Patrick DL, Perrin EB, et al. Evaluating quality-of-life and health status instruments: Development of scientific review criteria. Clinical Therapeutics. 1996;18:979–992. doi: 10.1016/S0149-2918(96)80054-3.ArticlePubMed
- 18. Mesbah M, Cole BF, Lee MT. Statistical methods for quality of life studies. 2002;Boston, Kluwer Academic Publishers.
- 19. Patrick DL, Chiang YP. Measurement of health outcomes in treatment effectiveness evaluations: Conceptual and methodological challenges. Medical Care. 2000;38:9 suppl. II14–II25. doi: 10.1016/S0149-2918(96)80054-3.PubMed
- 20. Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes/Metabolism Research and Reviews. 1999;15:205–218. doi: 10.1002/(SICI)1520-7560(199905/06)15:3<205::AID-DMRR29>3.0.CO;2-O.ArticlePubMed
- 21. Scientific Advisory Committee. Instrument review criteria. Medical Outcomes Trust Bulletin. 1995;3:I–IV.
- 22. Smith KW, Avis NE, Assmann SF. Distinguishing between quality of life and health status in quality of life research: A meta-analysis. Quality of Life Research. 1999;8:447–459. doi: 10.1023/A:1008928518577.ArticlePubMedPDF
- 23. Snoek FJ. Quality of life: A closer look at measuring patients' well-being. Diabetes Spectrum. 2000;13:24–28.
- 24. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30:473–483.ArticlePubMed
- 25. Watkins K, Connell CM. Measurement of health related QOL in diabetes mellitus. Pharmacoeconomics. 2004;22:1109–1126. doi: 10.2165/00019053-200422170-00002.ArticlePubMed
REFERENCES
Appendix
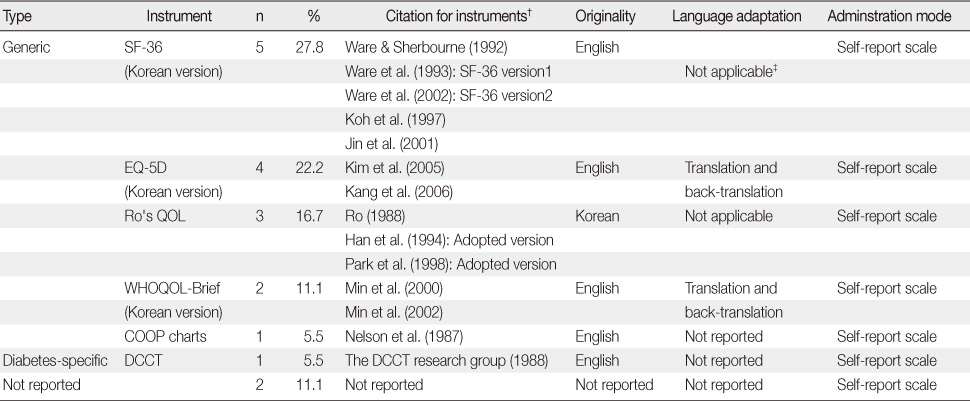
*The number of instruments used for the study was 18 because both SF-36 and EQ-5D were used in one study; †References for each citation were provided in appendix 1; ‡The Korean version is provided from QualityMetric Inc.
SF-36=Short form-36; EQ-5D=The EuroQoL-5Dimension; QOL=Quality of life; WHOQOL-Brief=World health organization QOL-Brief; COOP chart=The dartmouth-northern new England primary care cooperative information chart; DCCT=Diabetes control and complications trial.
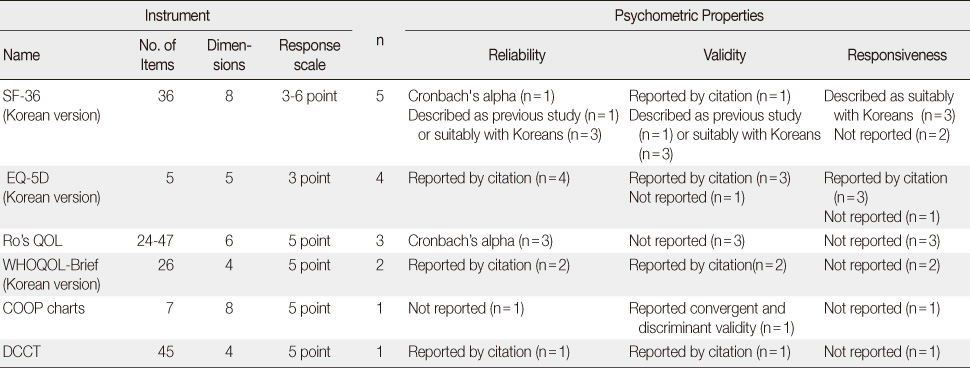
*The number of used instruments was 16 because in one study both SF-36 and EQ-5D were used, while for two studies instrument were not reported.
SF-36=Short Form-36; EQ-5D=The EuroQoL-5Dimension; QOL=Quality of life; WHOQOL-Brief=World health organization QOL-Brief; COOP chart=The dartmouth-northern new England primary care cooperative information chart; DCCT=Diabetes control and complications trial.
Figure & Data
REFERENCES
Citations

- The Characteristics related to Pulmonary Rehabilitation in Patients with Chronic Obstructive Pulmonary Disease: A Cross-sectional Study, Data from the Korea National Health and Nutrition Examination Survey 2015-2019.
Kyeongbong Lee
Physical Therapy Rehabilitation Science.2023; 12(3): 229. CrossRef - Factors affecting the health status of patients with type 2 diabetes mellitus receiving insulin treatments: A multi‐mediation path analysis
Kang Sun Lee, Hye Young Kim, Heung Young Jin
Journal of Clinical Nursing.2022; 31(9-10): 1285. CrossRef - Health-related Quality of Life Instrument With 8 Items for Use in Patients With Type 2 Diabetes Mellitus: A Validation Study in Korea
Juyoung Kim, Hyeon-Jeong Lee, Min-Woo Jo
Journal of Preventive Medicine and Public Health.2022; 55(3): 234. CrossRef - The Effects of the Level of Health Literacy and Self-care Activities on Quality of Life of Patients with Diabetes in Korea
Soo Jin Kang, Chanho Park
Journal of Korean Academy of Community Health Nursing.2020; 31(2): 189. CrossRef - Predictors of Health-Related Quality of Life in Korean Adults with Diabetes Mellitus
Mihyun Jeong
International Journal of Environmental Research and Public Health.2020; 17(23): 9058. CrossRef - Development and Evaluation of Allergic Rhinitis-Specific Quality of Life (ARSQOL) Scale for Adults
Hye-Sook Lee, Eunok Park
Journal of Korean Academy of Nursing.2016; 46(5): 675. CrossRef - Factors Related to Perceived Health Status in Patients with Type 2 Diabetes
Ang Li Won, Seung Hyun Yoo, Myoung Soon You
Korean Journal of Health Education and Promotion.2014; 31(3): 1. CrossRef - When Does the Quality of Life Improve after Rotator Cuff Repair?
Chul-Hyun Cho, Young Jae Lim
Journal of the Korean Orthopaedic Association.2013; 48(4): 281. CrossRef - Development and psychometric evaluation of a diabetes-specific quality-of-life (D-QOL) scale
Eun-Hyun Lee, Young Whee Lee, Kwan-Woo Lee, Dae Jung Kim, Soo-Kyung Kim
Diabetes Research and Clinical Practice.2012; 95(1): 76. CrossRef
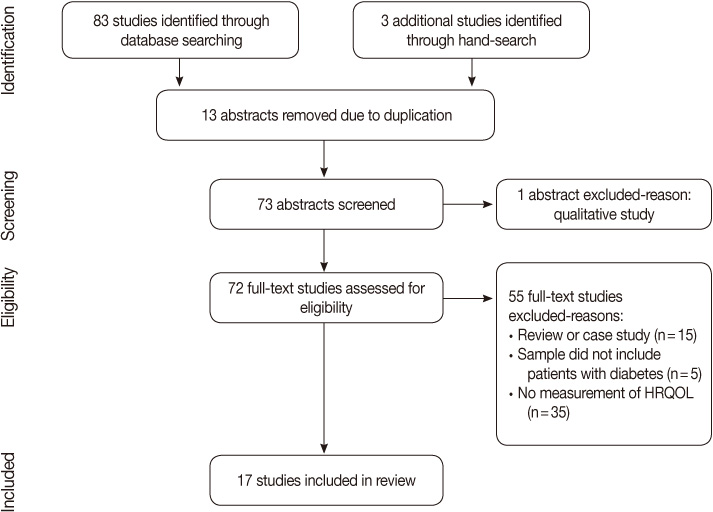
Figure 1
Characteristics of the Studies and Reported Conceptual Definitions, Taxonomy, and Attributes (N=17)
Citation and Application of Instruments Included for Korean Studies of Patients with Diabetes (N=18)*
*The number of instruments used for the study was 18 because both SF-36 and EQ-5D were used in one study; †References for each citation were provided in appendix 1; ‡The Korean version is provided from QualityMetric Inc.
SF-36=Short form-36; EQ-5D=The EuroQoL-5Dimension; QOL=Quality of life; WHOQOL-Brief=World health organization QOL-Brief; COOP chart=The dartmouth-northern new England primary care cooperative information chart; DCCT=Diabetes control and complications trial.
Reported Psychometric Properties of Instruments Used in Korean Studies of Patients with Diabetes (N=16)*
*The number of used instruments was 16 because in one study both SF-36 and EQ-5D were used, while for two studies instrument were not reported.
SF-36=Short Form-36; EQ-5D=The EuroQoL-5Dimension; QOL=Quality of life; WHOQOL-Brief=World health organization QOL-Brief; COOP chart=The dartmouth-northern new England primary care cooperative information chart; DCCT=Diabetes control and complications trial.
*The number of instruments used for the study was 18 because both SF-36 and EQ-5D were used in one study; †References for each citation were provided in appendix 1; ‡The Korean version is provided from QualityMetric Inc. SF-36=Short form-36; EQ-5D=The EuroQoL-5Dimension; QOL=Quality of life; WHOQOL-Brief=World health organization QOL-Brief; COOP chart=The dartmouth-northern new England primary care cooperative information chart; DCCT=Diabetes control and complications trial.
*The number of used instruments was 16 because in one study both SF-36 and EQ-5D were used, while for two studies instrument were not reported. SF-36=Short Form-36; EQ-5D=The EuroQoL-5Dimension; QOL=Quality of life; WHOQOL-Brief=World health organization QOL-Brief; COOP chart=The dartmouth-northern new England primary care cooperative information chart; DCCT=Diabetes control and complications trial.
 KSNS
KSNS
 E-SUBMISSION
E-SUBMISSION


 Cite
Cite