Articles
- Page Path
- HOME > J Korean Acad Nurs > Volume 53(4); 2023 > Article
- Research Paper Assessment of Risk Factors for Postoperative Delirium in Older Adults Who Underwent Spinal Surgery and Identifying Associated Biomarkers Using Exosomal Protein
- Wonhee Baek, JuHee Lee, Yeonsoo Jang, Jeongmin Kim, Dong Ah Shin, Hyunki Park, Bon-Nyeo Koo, Hyangkyu Lee
-
Journal of Korean Academy of Nursing 2023;53(4):371-384.
DOI: https://doi.org/10.4040/jkan.22146
Published online: August 31, 2023
2Mo-Im Kim Nursing Research Institute, College of Nursing, Yonsei University, Seoul, Korea
3Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea
4Department of Neurosurgery, Spine and Spinal Cord Institute, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
-
Corresponding author:
Hyangkyu Lee,
Email: hkyulee@yuhs.ac
Abstract
Purpose
With an increase in the aging population, the number of patients with degenerative spinal diseases undergoing surgery has risen, as has the incidence of postoperative delirium. This study aimed to investigate the risk factors affecting postoperative delirium in older adults who had undergone spine surgery and to identify the associated biomarkers.
Methods
This study is a prospective study. Data of 100 patients aged ≥ 70 years who underwent spinal surgery were analyzed. Demographic data, medical history, clinical characteristics, cognitive function, depression symptoms, functional status, frailty, and nutritional status were investigated to identify the risk factors for delirium. The Confusion Assessment Method, Delirium Rating Scale-R-98, and Nursing Delirium Scale were also used for diagnosing deliri-um. To discover the biomarkers, urine extracellular vesicles (EVs) were analyzed for tau, ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), neurofilament light, and glial fibrillary acidic protein using digital immunoassay technology.
Results
Nine patients were excluded, and data obtained from the remaining 91 were analyzed. Among them, 18 (19.8%) developed delirium. Differences were observed between partici-pants with and without delirium in the contexts of a history of mental disorder and use of benzodiazepines (p = .005 and p = .026, respectively). Tau and UCH-L1—concentrations of urine EVs—were comparatively higher in participants with severe delirium than that in partici-pants without delirium (p = .002 and p = .001, respectively).
Conclusion
These findings can assist clinicians in accurately identifying the risk factors before surgery, classifying high-risk patients, and predicting and detecting delirium in older patients. Moreover, urine EV analysis revealed that postoperative delirium following spinal surgery is most likely associated with brain damage.
Published online Aug 17, 2023.
https://doi.org/10.4040/jkan.22146
Assessment of Risk Factors for Postoperative Delirium in Older Adults Who Underwent Spinal Surgery and Identifying Associated Biomarkers Using Exosomal Protein
Abstract
Purpose
With an increase in the aging population, the number of patients with degenerative spinal diseases undergoing surgery has risen, as has the incidence of postoperative delirium. This study aimed to investigate the risk factors affecting postoperative delirium in older adults who had undergone spine surgery and to identify the associated biomarkers.
Methods
This study is a prospective study. Data of 100 patients aged ≥ 70 years who underwent spinal surgery were analyzed. Demographic data, medical history, clinical characteristics, cognitive function, depression symptoms, functional status, frailty, and nutritional status were investigated to identify the risk factors for delirium. The Confusion Assessment Method, Delirium Rating Scale-R-98, and Nursing Delirium Scale were also used for diagnosing delirium. To discover the biomarkers, urine extracellular vesicles (EVs) were analyzed for tau, ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), neurofilament light, and glial fibrillary acidic protein using digital immunoassay technology.
Results
Nine patients were excluded, and data obtained from the remaining 91 were analyzed. Among them, 18 (19.8%) developed delirium. Differences were observed between participants with and without delirium in the contexts of a history of mental disorder and use of benzodiazepines (p = .005 and p = .026, respectively). Tau and UCH-L1—concentrations of urine EVs—were comparatively higher in participants with severe delirium than that in participants without delirium (p = .002 and p = .001, respectively).
Conclusion
These findings can assist clinicians in accurately identifying the risk factors before surgery, classifying high-risk patients, and predicting and detecting delirium in older patients. Moreover, urine EV analysis revealed that postoperative delirium following spinal surgery is most likely associated with brain damage.
INTRODUCTION
Degenerative spinal diseases are increasing worldwide [1], as is the number of surgeries; degenerative spinal surgeries increased by 62.3% from 2004 to 2015 in the USA [2] and by 54% from 1999 to 2013 in Norway [3]. Moreover, the number of older people who have undergone spinal surgery has steadily risen over the years [1, 3, 4]. Postoperative complications after spinal surgery include cardiovascular, respiratory, and wound-related complications; inpatient falls; and postoperative delirium [4]. It is worth noting that the incidence of postoperative complications of spinal surgery has remained stable from 2006 to 2016, whereas that of postoperative delirium has risen in the USA [4].
Delirium is a state characterized by a sudden change in the consciousness level, which may be accompanied by loss of brain function with attention deficit, memory loss, and abnormal behavior [5]. Previous studies have revealed that 8% to 40% of patients who had undergone spinal surgery had postoperative delirium at age 70 [6]. Postoperative delirium was associated with complications including wound infection, deep vein thrombosis, pneumonia, and pressure ulcers [7]. Furthermore, in the long term, it can lead to impaired functional recovery, decreased quality of life, declined cognitive function, and increased mortality [8].
Age is a significant risk factor for postoperative delirium and the likelihood of developing delirium increases in older patients when one or two risk factor precipitants are added [8]. The predisposing factors for delirium after spinal surgery in older adults include opioid use, hypertension, cerebrovascular disease, and pulmonary disease. The precipitating factors include the site, method, duration of surgery, and infused intravenous fluid volume [6]. A comprehensive geriatric assessment and patient-specific interventions based on preoperative assessment can reduce the incidence of postoperative delirium, related complications, and length of hospital stay [9, 10]. Therefore, it is necessary to identify delirium risk factors to stratify the risk groups and tailor interventions to the patient’s characteristics.
In particular, pain, frailty, activities of daily living, and the nutritional status of older adults undergoing orthopedic surgery affect postoperative complications [11]. However, previous studies of spinal surgery patients have only investigated fragmentary risk factors for postoperative delirium, such as disease-related and laboratory data and surgery-related factors [6]. Therefore, it is necessary to determine whether certain characteristics of older adults who have had spinal surgery affect delirium. The treatment of delirium is centered on preoperative assessment of the patient [11] and prevention and management of the disease [10]. However, the pathophysiology of postoperative delirium remains unclear [8], and there is no solid evidence of medical treatment [10]. Hence, investigating biomarkers is crucial as they can help diagnose and understand this condition.
Previous studies that have explored the biomarkers of postoperative delirium were based on the relatively new hypothesis that the condition originated from brain injury [12, 13, 14]. Digital enzyme-linked immunosorbent assay is highly sensitive and can accurately quantify low concentrations of proteins or peptides with excellent reproducibility, which makes it advantageous for identifying biomarkers [15, 16]. Plasma tau [13, 14] and neurofilament light (NFL) [12] have been identified as biomarkers of delirium using Single-Molecule Array (Simoa) analysis. However, collecting blood samples from older adults and patients experiencing hyperactive delirium is challenging because of low blood pressure, dehydration, and loss of vein patency and elasticity. Thus, biomarkers must be identified using samples obtained through minimally invasive techniques, such as urine sampling.
Extracellular vesicles (EVs) are 40~100 nm extracellular endoplasmic reticulum that retains cell characteristics and information; they have recently garnered attention as potential biomarkers of cancer and neurodegenerative diseases [17, 18]. EVs can easily be obtained from blood [18], saliva [19], cerebrospinal fluid [17], and urine [20] samples. They further have the advantages of stability, specificity, and immediate reactivity. In addition, EVs can cross the blood–brain barrier and exchange substances through the cell membrane [17], eliciting details about the state of the brain and body during delirium. This study aimed to explore the risk factors for postoperative delirium in patients aged ≥ 70 years who underwent spinal surgery. In doing so, it identified biomarkers from urinary EVs using digital immunoassay technology.
METHODS
1. Study design
A prospective design was used for this study to examine the risk factors of postoperative delirium and discover biomarkers.
2. Setting and sample
A study that used EVs to identify diagnostic biomarkers in Alzheimer’s disease [17] predicted that 10 patients with delirium would be needed for adequate statistical power. Applying the predicted 12.5% incidence of postoperative delirium in patients aged ≥ 70 years who underwent spinal surgery, this study required 80 participants. Assuming a 20% dropout rate, 100 participants were finally recruited. The researchers recruited patients at a tertiary hospital in Seoul between October 21, 2019, and May 19, 2020. The inclusion criteria were as follows: 1) patients aged ≥ 70 years and 2) patients undergoing cervical, thoracic, and lumbar spine surgery (including spinal fusion, laminectomy, laminoplasty, foraminotomy, and discectomy). The exclusion criteria were as follows: 1) patients with cognitive decline according to the Mini-Mental State Examination for Dementia Screening (MMSE-DS) criteria; 2) patients with a diagnosis of a malignant tumor in the last five years; 3) patients with a scheduled surgery time < 2 hours or minimally invasive surgery; 4) patients with a history of cranial nerve disease, cerebral hemorrhage, stroke, dementia, or Parkinson’s disease; and 5) patients with alcoholism or drug addiction.
3. Measurements
1) Demographic data, medical history, and clinical characteristics
Demographic data and medical histories such as age, sex, level of education, history of delirium, comorbidities, and prescribed drugs were acquired from participants using a standardized questionnaire. The missing data and surgical characteristics were also obtained from the electronic medical records.
2) Assessment of delirium
The Confusion Assessment Method (CAM) [21] and Nursing Delirium Screening Scale (Nu-DESC) [22] were used for diagnosing delirium. The sensitivity and specificity of CAM for delirium were over 94% and 90%, respectively [21]. It has been routinely used in diagnosing delirium in spinal surgery patients, and its validity has been proven [6]. Nu-DESC can assess changes in the five delirium-related signs and symptoms. It was developed so that busy nurses could readily use it to diagnose delirium quickly [22], and its sensitivity and validity have been verified in South Korea [23]. Furthermore, the Delirium Rating Scale-R-98 (DRS-R-98) [24] was used to classify the severity and episodes of delirium; a previous study with the same participants has demonstrated its validity [25]. In addition, nurses, physicians, and caregivers were interviewed about the patient’s condition; and medical records were comprehensively reviewed to make a diagnosis. Three subtypes were identified according to the characteristics of delirium symptoms [8]: the hyperactive type refers to a state with an excessive level of psychomotor activity, resulting in agitation, wandering, and hallucination; the hypoactive type refers to a state with a low level of psychomotor activity, causing unawareness, withdrawal, and decreased activity; and the mixed type is a combination of both [8].
3) Other tests to assess participants
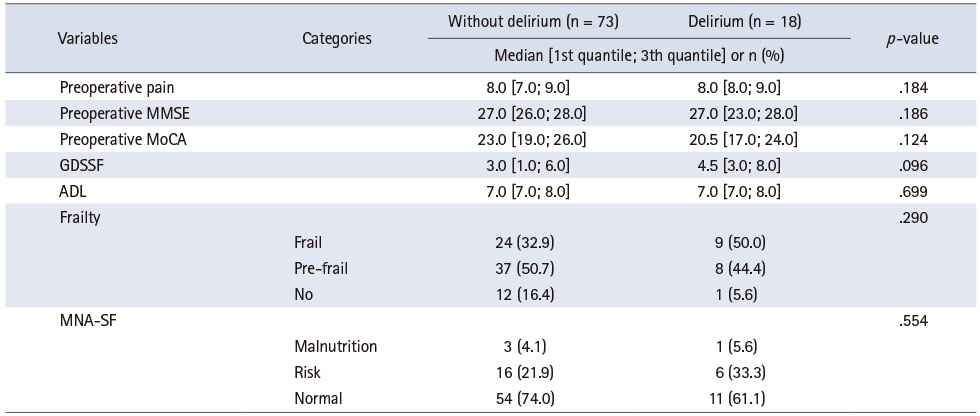
The Korean version of MMSE-DS [26] was used to exclude participants with cognitive decline; its Cronbach’s alpha is 0.83, the inter-rater agreement is 0.99, and the test-retest reliability is 0.94 [26]. The Korean version of the Montreal Cognitive Assessment [27] was used to measure cognitive function; its Cronbach’s alpha is 0.84, and the test-retest reliability is 0.85. The total score for both instruments is 30 points each, and the higher the score, the higher the cognitive function. We also assessed depression symptoms using the Geriatric Depression Scale Short Form [28], consisting of a 15-item questionnaire with a total score of 15; the higher the score, the higher the level of depression. Its splithalf reliability is 0.85, and the test-retest reliability is 0.90 [28]. Functional status was measured using the Korean Activities of Daily Living [29], which has a Cronbach’s alpha of 0.937. It consists of a seven-item questionnaire with a total score of 21, and the higher the score, the weaker the function. In terms of frailty, the measure of the Korean version of the fatigue, resistance, ambulation, illnesses, and loss of weight (FRAIL) scale [30] was used. A score of 0 out of 5 indicates robustness, 1 or 2 indicates prefrailty, and 3 or more indicates frailty. Its sensitivity is 0.90, and specificity in the ability to discriminate prefrail or frail participants compared to the robust ones is 0.33 [30]. Nutritional status was measured using the Mini Nutritional Assessment Short Form [31], which consists of a six-item questionnaire and is highly sensitive compared to the full Mini Nutritional Assessment. On a total of 14 points, a score of 12 or higher indicates normal nutritional status, while that of 8 to 11 points indicates a risk for malnutrition, and that of 0 to 7 points indicates malnutrition.
4. Data collection
When the participants visited the hospital at least two weeks before surgery, the researcher was offered a separate area to explain the study and obtain informed consent for participation. Considering the possibility that the patients might complain of discomfort or pain while sitting, a recliner chair was provided for comfort. Furthermore, the researcher ended the survey when the patient complained of discomfort because those aged ≥ 70 years may have vision or hearing difficulties. The researcher collected data on the general characteristics, medical history, cognitive function, depression symptoms, functional status, frailty, and nutritional status. Urine was collected within 3 hours after surgery and stored in a -80℃ freezer. The researcher visited the patients daily at 8:00 am and 6:00 pm. Delirium was diagnosed using comprehensive judgment, diagnostic tools, medical records every 8 hours, and daily interviews with the medical staff in charge and the caregivers. If it was unclear whether a patient’s condition met the criteria for diagnosis, we consulted the psychiatrist and anesthesiologist to reach an agreement.
5. Exosomal biomarker quantification
Protein biomarkers from urinary EVs were measured using Simoa HD-x Analyzer (Quanterix Corporation, Lexington, MA, USA), which allows multiplex detection with single-molecule sensitivity. Simoa technology is a highly sensitive digital immunoassay that accurately measures candidate biomarkers at low concentrations in biofluids such as blood, urine, or cerebrospinal fluid. Four neurological markers—total tau, ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), glial fibrillary acidic protein (GFAP), and NFL—were quantified using the Simoa Neurology 4-plex assay kit (Quanterix; Cat. No. 102153, Billerica, MA, USA) [32]. An exosomal protein analysis was conducted on all participants with delirium. We selected and analyzed 54 participants without delirium as a control group. We matched these participants to those with delirium based on sex, age, type of surgery, and surgical time. All samples were analyzed in duplicate, and technicians running the assays were blinded to all data. Data were not used if the intra-assay or inter-assay performance was higher than 20%, which occurred in less than 5% of the samples. The lower limits of quantification were 0.241 pg/mL, 0.467 pg/mL, 5.45 pg/mL, and 0.053 pg/mL for NFL, GFAP, UCH-L1, and tau, respectively.
6. Data analysis
Depending on the variable characteristics, the participants’ general characteristics were reported as frequencies, percentages, averages, standard deviations, medians, first quartiles, or third quartiles. Subsequently, the differences between the participants with and without delirium were analyzed using an independent t-test, a Wilcoxon rank-sum test, a Fisher’s exact test, and a chi-square test. Outlier values of tau, UCH-L1, GFAP, and NFL concentrations were calculated using the interquartile range rule formula and presented in quartiles. Wilcoxon rank-sum test was used to confirm the difference in the concentrations of tau, UCH-L1, GFAP, and NFL between the participants with and without delirium. As the severity of delirium varied widely, protein levels in six participants with the highest severity of 30% delirium and six matched participants who did not have delirium were analyzed using the Wilcoxon rank-sum test. The variables used for matching were age, sex, length of surgery, and type of surgery. All data were analyzed using the R program version 4.0.0 (https://www.r-
7. Ethical consideration
This study was approved by the Institutional Review Board of Yonsei University Health System (IRB No. 4-2019-0654). The study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all study participants.
RESULTS
1. Incidence, duration, and degree of delirium
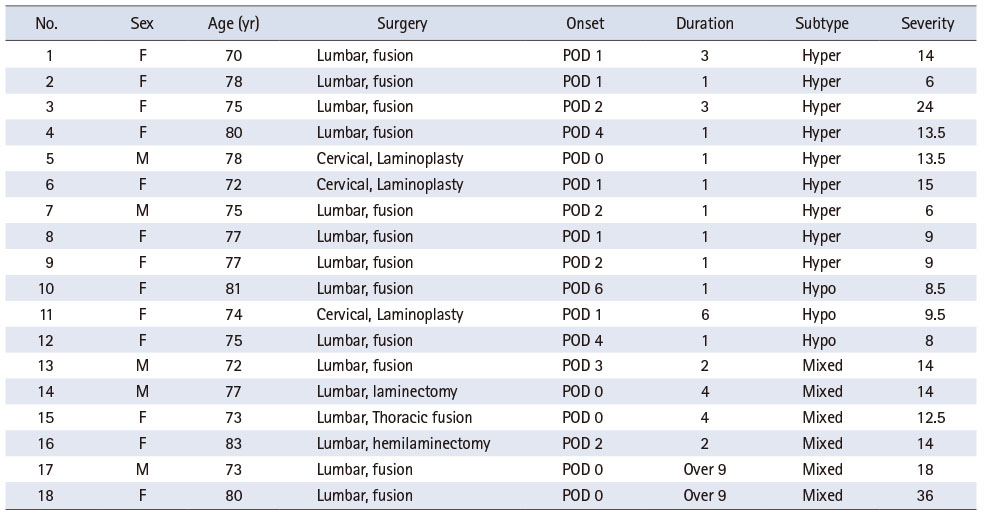
Data from 100 patients aged ≥ 70 years who underwent spinal surgery were analyzed. Among them, nine were excluded because of decline (n = 2), surgical complications (n = 2), and surgery cancellation (n = 5). Thus, 91 patients formed the study population; 18 (19.8%) developed postoperative delirium. Of these 18 patients, 13 (72.2%) were women. Further, the average age of the study population was 76.5 ± 3.3 years. Fifteen of these patients (83.3%) underwent lumbar surgery, and thirteen (72.2%) had fusion surgeries. According to the delirium subtype, nine patients were classified as hyperactive (50%), three as hypoactive (17%), and six (33%) as mixed. The duration of delirium was more than nine days in two patients. Delirium severity scores ranged between 6~36 points (Table 1).
Table 1
Incidence, Duration, and Degree of Delirium
2. Characteristics of participants with or without delirium
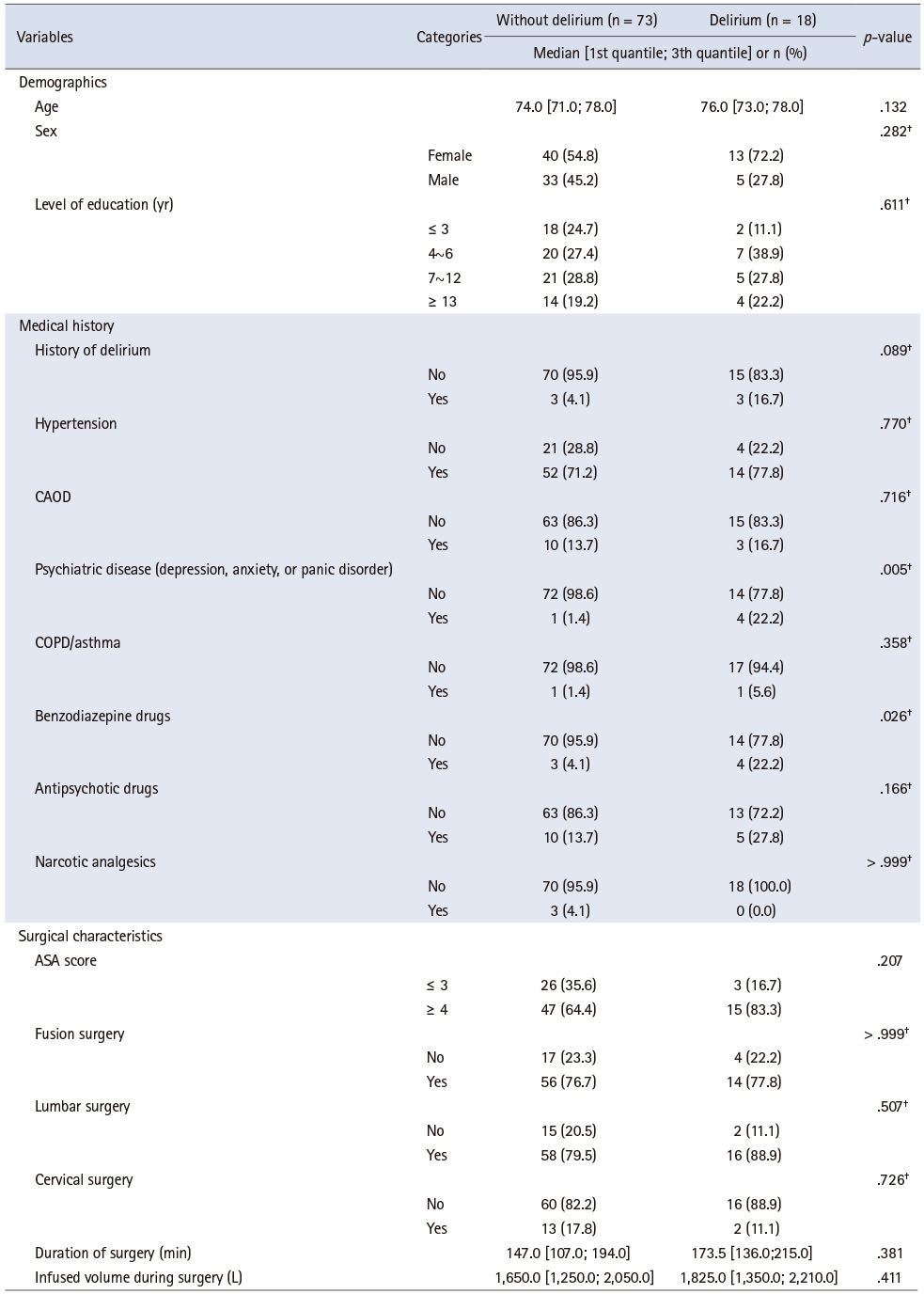
Table 2 shows the differences in the demographics, medical history, and surgical characteristics of participants with and without delirium. There were no differences based on age, sex, final education, history of delirium and diseases, use of antipsychotic drugs, use of narcotic analgesics, physical classification, surgical site and duration, and intraoperative infused volume between the two groups. Differences in the history of psychiatric disorder (depression, anxiety, or panic disorder) and use of benzodiazepines were noted between participants with and without delirium (p = .005 and p = .026, respectively). Finally, no significant differences in preoperative pain, cognitive function, depression symptoms, functional status, frailty, or nutritional status were noted (Table 3).
Table 2
Demographic, Medical History, and Surgical Characteristics of Patients with and without Delirium
Table 3
Variables to Understand the Participants
3. Tau, UCH-L1, GFAP, and NFL of urinary EVs
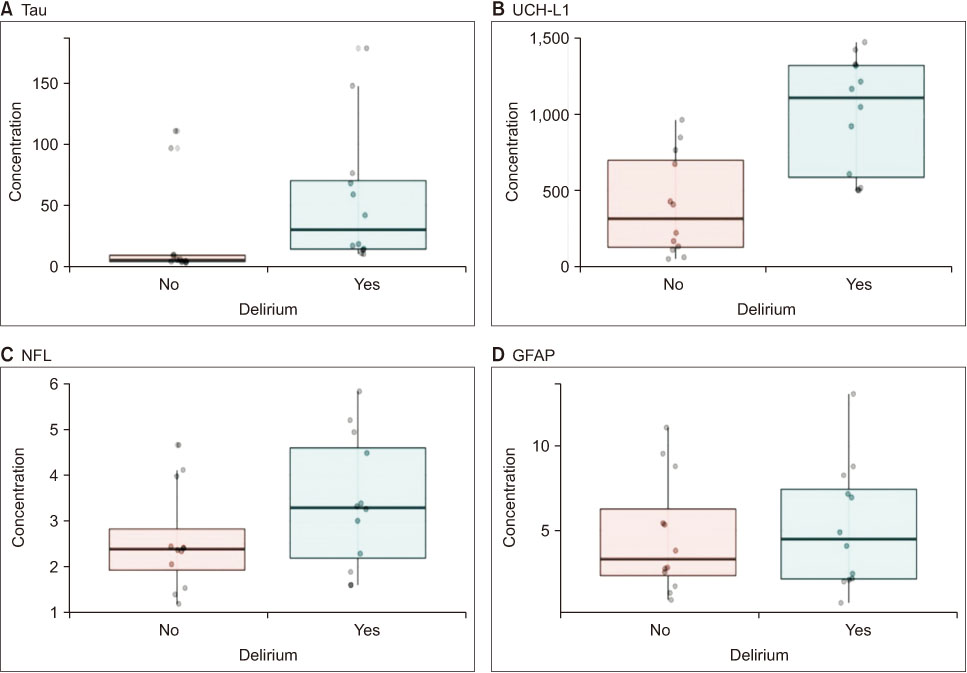
Three samples were excluded from the measurement of tau, while one sample was excluded from each of the UCH-L1, GFAP, and NFL measurements, because of inadequate data. Exosome characteristics of urinary EVs are presented in Supplementary Figure 1. The concentrations of tau, UCH-L1, GFAP, and NFL, excluding outliers, showed no significant difference between participants with and without delirium (Supplementary Table 1 and Supplementary Figure 2). The concentrations of tau and UCH-L1 were significantly different between participants in the top 30% of delirium severity, and six participants without delirium were matched with this top 30% (Figure 1 and Table 4).
Figure 1
UCH-L1 = Ubiquitin carboxyl-terminal hydrolase L1; NFL = Neurofilament light; GFAP = Glial fibrillary acid protein.
Neurological biomarker concentrations in urinary exosomes.
Box plot of (A) tau, (B) UCH-L1, (C) GFAP, and (D) NFL of urinary extracellular vesicles; participants in the top 30% of delirium severity. Tau, UCH-L1, NFL, and GFAP (N = 12) assays were measured twice per sample.
Table 4
Tau, UCH-L1, NFL, and GFAP of Urinary EVs; Participants in the Top 30% of Delirium Severity (N = 12)
DISCUSSION
This prospective cross-sectional study attempted to identify the risk factors and biomarkers for postoperative delirium using EVs in patients aged ≥ 70 years who underwent spinal surgery. The incidence of delirium among the study participants was 19.8%, which was higher than that reported by another South Korean study [33]. This difference may be attributed to the prospective nature of the present study and the fact that delirium was not diagnosed until discharge. Most participants in our study developed delirium within four postoperative days. However, the timing of onset varied, consistent with the findings of a previous study [8]. It suggests that nurses should pay attention to any signs of delirium in older patients after surgery until discharge. In an American study [25] conducted on the same target population, the incidence of delirium was reported to be as high as 40%. The incidence of delirium in our study was much lower because we strictly excluded patients with a high possibility of delirium, such as patients with cognitive impairment and cancer. Therefore, this study explored the risk factors for postoperative delirium exclusively.
The present study’s risk factors for delirium included a history of benzodiazepine use. These findings were consistent with those of previous studies [34, 35]. In particular, a cognitive disorder in older adults increased in long-term benzodiazepine users than in short-term users [36]. As of the many adverse effects of benzodiazepine use, such as cognitive decline and dependence, the South Korean government began to restrict benzodiazepine prescriptions in 2010; however, long-term prescriptions remain prevalent [37]. Deciding whether to continue taking benzodiazepines or discontinue them before surgery is important in postoperative delirium. Omich et al. [34] reported that the group that discontinued benzodiazepines before surgery had a higher risk of delirium than those that never used or continued using them. However, most drugs, including benzodiazepines, are abruptly stopped before surgery, and only essential drugs such as anti-hypertensive are administered; thus, in most cases, there is a possibility that the side effects of abruptly stopping benzodiazepines manifested postoperatively in patients who had been taking these for a prolonged period. Given the results, nurses need to carefully evaluate the types and patterns of medications taken by patients, particularly psychiatric medications.
Moreover, among orthopedic surgery patients, the incidence of postoperative delirium increased with the severity of depression, and the depression symptoms were associated with the pathology of Alzheimer’s disease as detected in the cerebrospinal fluid [38]. Depression has been associated with decreased overall perception, cognitive decline, episodic memory, performance, and processing speed [39]. Therefore, if delirium occurs after surgery in a patient with depression, the chances of them developing cognitive dysfunction should be considered, and regular follow-up examinations should be performed. Furthermore, preoperative anxiety has been reported to increase agitation after surgery [40]. A previous study in Japan reported that preoperative anxiety treatment effectively reduced postoperative delirium [41]. Contrarily, a study of older patients who had cardiac surgery reported that postoperative delirium was not associated with anxiety and depression symptoms [42]. Additionally, as the number of participants with each disorder was small in this study, the individual disorders could not be analyzed separately. Further validation studies are required to better understand the relationship between postoperative delirium and psychiatric disorders.
Using urine EVs, this study explored the biomarkers associated with delirium through a concentration analysis of tau, UCH-L1, NFL, and GFAP proteins, which indicate nerve damage. EV biomarkers of Alzheimer’s disease have previously been discovered through next-generation deep sequencing or microarrays of serum and cerebrospinal fluid [17, 18]. The Simoa analysis technique used in this study for postoperative delirium was similar to that used by Fong et al. [12] and Ballweg et al. [14]. Most participants with cognitive impairment, including delirium, have comparatively weaker blood vessels or require minimally invasive treatment to prevent infection. As delirium occurs episodically, no difficulties should be encountered in terms of the time of sample collection. Therefore, we determined that the readily accessible urine sample was suitable for biomarker screening. Moreover, urine undergoes little protein degradation in the urinary tract or bladder and can be stored for a longer period of time [43]. As long as a urine catheter is inserted in patients, urine can be collected at any time, which can help detect the changes in the patient’s cells at the time of the onset of delirium. Therefore, EV samples in urine were ideal for identifying the biomarkers of delirium after spinal surgery.
UCH-L1 concentration was high among the participants with the top 30% of delirium severity (p < .001). UCH-L1 plays a vital role in the proteasome system by removing misfolded or oxidized proteins [44]. Contrary to our findings, a study conducted on 427 intensive care unit patients showed that high plasma UCH-L1 concentrations were related to a lower incidence of delirium [45]. In addition, Ballweg et al. [14] reported no relationship between postoperative delirium and UCH-L1 concentration. Differences in the results may be attributed to the dissimilarities between the participants of the present study and those of Hayhurst et al. [45] and Ballweg et al. [14]. Furthermore, the relationship between UCH-L1 and delirium remains controversial [45]. Therefore, more research is necessary to verify the relationship between UCH-L1 and postoperative delirium. Previous studies on patients with brain injuries [46, 47] have shown a positive correlation between UCH-L1 concentration and brain injury after surgery. UCH-L1 has been reported to better predict the clinical prognosis of patients after undergoing brain damage, as compared to S100B, which is an indicator of traumatic brain injury [46]. Unlike delirium caused by other factors, postoperative delirium may cause brain injury due to surgery-induced tissue damage, systemic inflammatory reaction, and oxidative metabolism [48]. Therefore, further repetition studies are needed based on the premise that surgery might cause brain damage.
Tau is a protein indicating neurodegeneration; an increase in tau concentration has been associated with dementia, stroke, and traumatic brain injury [49]. Further, on comparing the top 30% of participants with severe delirium and those without delirium, tau concentration was found to be higher among the participants with delirium (p < .002), which was consistent with previous studies [13, 14]. From preoperative to postoperative day 1, plasma tau increased more significantly in patients with postoperative delirium and correlated with delirium severity [14]. In cardiac surgery, preoperative tau levels were elevated in patients with delirium than that in those without delirium [13]. Based on our findings and the results of previous studies, it can be concluded that patients with severe delirium may have a higher risk of neurodegeneration and brain injury. However, since the urine used in the analysis was collected only after surgery on the same day, differences in tau concentrations before and after surgery were not confirmed. Thus, surgery does not necessarily cause brain injury. Since a causal relationship could not be established due to the experimental design, further studies are needed to confirm this aspect. In addition, since tau is already used as a biomarker in the research on cognitive dysfunction such as dementia, it may potentially serve as a significant biomarker in determining the relationship between postoperative delirium and cognitive dysfunction.
This study provides new insights into the influencing factors of postoperative delirium through a comprehensive assessment and urine exosome analysis of spinal surgery patients aged ≥ 70 years. Prior to surgery, nurses should conduct a thorough drug assessment and help patients on benzodiazepines to consider a prescription change or encourage them to choose non-pharmacological treatment methods such as psychological support and tapering education [50]. In addition, nurses can use the history of benzodiazepine use and psychiatric disorders to classify patients at risk for postoperative delirium. Since the onset time of delirium varied in this study, it is necessary to consider the possibility of delirium in high-risk patients until discharge and to monitor these patients closely. This study confirmed that postoperative delirium might be related to brain damage through digital immunoassay of urine exosomes. Therefore, nurses must explain to patients the possibility of cognitive decline in postoperative delirium so that they can continue to follow up on cognitive function testing. Along with this nursing implication, training geriatric nurses to professionally use comprehensive assessments may be valuable. Comprehensive geriatric assessments, non-pharmacological interventions, and early detection of delirium by the nurse are essential in managing postoperative delirium in older adults. Furthermore, a multidisciplinary approach with anesthesiologists and surgeons should be included to remove or modify the risk factors of postoperative delirium during the perioperative period [6].
This study has some limitations. First, because the number of patients diagnosed with depression, anxiety, and panic disorder was small, the data were merged and analyzed as a psychiatric disorder. Therefore, it was not possible to explore the relevance of delirium according to the exact diagnosis. Second, since protein analysis in urine EVs did not involve measurement of the preoperative concentration, a pre and postoperative comparison could not be made. Therefore, a causal relationship to explain whether the difference was surgically induced, could not be established. Third, the distribution of delirium severity scores differed for each participant, and the number of patients with severe delirium was relatively small. Fourth, an analysis of neuronal EVs isolated from urine was not possible because the amount of extracted EVs was small. Further studies analyzing preoperative samples are necessary to provide the basis for causal reasoning by confirming whether delirium was due to brain damage, which can occur before or after surgery.
CONCLUSION
In summary, this study was conducted to investigate the risk factors associated with postoperative delirium in older adults aged ≥ 70 years who underwent spinal surgery and to identify the biomarkers associated with delirium by measuring tau, UCH-L1, GFAP, and NFL protein concentrations in urine EVs using digital immunoassay analysis. Our findings can assist healthcare providers in accurately investigating risk factors associated with delirium before surgery and help identify high-risk patients. In addition, tau and UCH-L1 may be used as potential biomarkers for postoperative delirium in elderly patients prior to surgery. However, because this study presents the results from only 91 patients who underwent spinal surgery in South Korea, a follow-up study with larger sample size and other populations is needed to generalize these to the global population.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online at https://doi.org/10.4040/jkan.22146.
Exosome characteristics of urinary extracellular vesicles. (A) EVs isolated from urine examined by size and concentration using Nanoparticle Tracking Analysis. The red line represents the error of means of the triplicate measurements; the black line represents the curve fitting. (B) Quantification of EV size and concentration.Supplementary Figure 1
Box plot of Tau, UCH-L1, GFAP, and NFL of urinary extracellular vesicles. (A) Tau, (B) UCH-L1, (C) GFAP, and (D) NFL assays were measured twice per sample and outliers were removed.Supplementary Figure 2
Concentrations of Tau, UCH-L1, NFL, and GFAPSupplementary Table 1
This manuscript is a condensed form of the first author’s doctoral dissertation from Yonsei University. Year of 2021.
This work was presented at the International Nursing Conference, Oct. 2021, Seoul, Republic of Korea.
CONFLICTS OF INTEREST:The authors declare no conflicts of interest.
FUNDING:This study was supported by The Health Fellowship Foundation to Baek W. and the National Research Foundation of Korea (NRF) grant funded by the Korea government (No. 2022R1A2B5B01002485) to Lee H.
AUTHOR CONTRIBUTIONS:
Conceptualization or/and Methodology: Baek W & Lee JH & Jang Y & Kim J & Shin DA & Park H & Koo BN & Lee H.
Data curation or/and Analysis: Baek W & Park H. (exosomes)
Funding acquisition: Baek W & Lee H.
Investigation: Baek W & Koo BN & Lee H.
Project administration or/and Supervision: Baek W & Lee JH & Jang Y & Kim J & Shin DA & Koo BN & Lee H.
Resources or/and Software: Baek W.
Validation: Baek W & Park H.
Visualization: Baek W & Park H.
Writing original draft or/and Review & editing: Baek W & Lee JH & Jang Y & Kim J & Shin DA & Park H & Koo BN & Lee H.
ACKNOWLEDGEMENTS
None.
DATA SHARING STATEMENT
Please contact the corresponding author for data availability.
References
-
Saller T, Petzold A, Zetterberg H, Kuhle J, Chappell D, von Dossow V, et al. A case series on the value of tau and neurofilament protein levels to predict and detect delirium in cardiac surgery patients. Biomedical Papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia 2019;163(3):241–246. [doi: 10.5507/bp.2019.043]
-
-
Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius Å, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clinical Chemistry and Laboratory Medicine 2016;54(10):1655–1661. [doi: 10.1515/cclm-2015-1195]
-
-
Riancho J, Vázquez-Higuera JL, Pozueta A, Lage C, Kazimierczak M, Bravo M, et al. MicroRNA profile in patients with Alzheimer’s disease: Analysis of miR-9-5p and miR-598 in raw and exosome enriched cerebrospinal fluid samples. Journal of Alzheimer’s Disease 2017;57(2):483–491. [doi: 10.3233/JAD-161179]
-
-
Cheng L, Doecke JD, Sharples RA, Villemagne VL, Fowler CJ, Rembach A, et al. Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Molecular Psychiatry 2015;20(10):1188–1196. [doi: 10.1038/mp.2014.127]
-
-
Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: Comparison with the delirium rating scale and the cognitive test for delirium. Journal of Neuropsychiatry and Clinical Neurosciences 2001;13(2):229–242. [doi: 10.1176/jnp.13.2.229]Erratum in: Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13(3):433.
-
-
Kang Y, Park J, Yu KH, Lee BC. A reliability, validity, and normative study of the Korean-Montreal Cognitive Assessment (K-MoCA) as an instrument for screening of vascular cognitive impairment (VCI). Korean Journal of Clinical Psychology 2009;28(2):549–562. [doi: 10.15842/kjcp.2009.28.2.013]
-
-
Kee BS. A preliminary study for the standardization of geriatric depression scale short form-Korea version. Journal of Korean Neuropsychiatric Association 1996;35(2):298–307.
-
-
Won CW, Yang KY, Rho YG, Kim SY, Lee EJ, Yoon JL, et al. The development of Korean activities of daily living (K-ADL) and Korean instrumental activities of daily living (K-IADL) scale. Journal of the Korean Geriatrics Society 2002;6(2):107–120.
-
-
Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. Journal of Nutrition, Health & Aging 2009;13(9):782–788. [doi: 10.1007/s12603-009-0214-7]
-
-
Omichi C, Ayani N, Oya N, Matsumoto Y, Tanaka M, Morimoto T, et al. Association between discontinuation of benzodiazepine receptor agonists and post-operative delirium among inpatients with liaison intervention: A retrospective cohort study. Comprehensive Psychiatry 2021;104:152216 [doi: 10.1016/j.comppsych.2020.152216]
-
-
Chan CK, Sieber FE, Blennow K, Inouye SK, Kahn G, Leoutsakos JS, et al. Association of depressive symptoms with postoperative delirium and CSF biomarkers for Alzheimer’s disease among hip fracture patients. American Journal of Geriatric Psychiatry 2021;29(12):1212–1221. [doi: 10.1016/j.jagp.2021.02.001]
-
-
Wada S, Inoguchi H, Hirayama T, Matsuoka YJ, Uchitomi Y, Ochiai H, et al. Yokukansan for the treatment of preoperative anxiety and postoperative delirium in colorectal cancer patients: A retrospective study. Japanese Journal of Clinical Oncology 2017;47(9):844–848. [doi: 10.1093/jjco/hyx080]
-
-
Detroyer E, Dobbels F, Verfaillie E, Meyfroidt G, Sergeant P, Milisen K. Is preoperative anxiety and depression associated with onset of delirium after cardiac surgery in older patients? A prospective cohort study. Journal of the American Geriatrics Society 2008;56(12):2278–2284. [doi: 10.1111/j.1532-5415.2008.02013.x]
-
-
Papa L, Lewis LM, Silvestri S, Falk JL, Giordano P, Brophy GM, et al. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. Journal of Trauma and Acute Care Surgery 2012;72(5):1335–1344. [doi: 10.1097/TA.0b013e3182491e3d]
-
 KSNS
KSNS
 E-SUBMISSION
E-SUBMISSION

 Cite
Cite